
Hydroxylysine (Hyl) is an amino acid with the molecular formula C6H14N2O3. It was first discovered in 1921 by Donald Van Slyke as the 5-hydroxylysine form. It arises from a post-translational hydroxy modification of lysine. It is most widely known as a component of collagen.
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.

The enzyme citrate synthase E.C. 2.3.3.1 ] exists in nearly all living cells and stands as a pace-making enzyme in the first step of the citric acid cycle. Citrate synthase is localized within eukaryotic cells in the mitochondrial matrix, but is encoded by nuclear DNA rather than mitochondrial. It is synthesized using cytoplasmic ribosomes, then transported into the mitochondrial matrix.

In enzymology, a L-lysine 6-monooxygenase (NADPH) (EC 1.14.13.59) is an enzyme that catalyzes the chemical reaction
In enzymology, a saccharopine dehydrogenase (NAD+, L-glutamate-forming) (EC 1.5.1.9) is an enzyme that catalyzes the chemical reaction
In enzymology, a saccharopine dehydrogenase (NADP+, L-lysine-forming) (EC 1.5.1.8) is an enzyme that catalyzes the chemical reaction
In enzymology, an isochorismatase (EC 3.3.2.1) is an enzyme that catalyzes the chemical reaction
The enzyme acetolactate decarboxylase (EC 4.1.1.5) catalyzes the chemical reaction
In enzymology, an aerobactin synthase (EC 6.3.2.39) is an enzyme that catalyzes the chemical reaction
In enzymology, an indoleacetate—lysine synthetase (EC 6.3.2.20) is an enzyme that catalyzes the chemical reaction
In enzymology, an arylamine N-acetyltransferase is an enzyme that catalyzes the chemical reaction
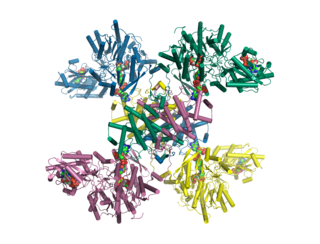
ATP citrate synthase (also ATP citrate lyase (ACLY)) is an enzyme that in animals represents an important step in fatty acid biosynthesis. By converting citrate to acetyl-CoA, the enzyme links carbohydrate metabolism, which yields citrate as an intermediate, with fatty acid biosynthesis, which consumes acetyl-CoA. In plants, ATP citrate lyase generates cytosolic acetyl-CoA precursors of thousands of specialized metabolites, including waxes, sterols, and polyketides.
In enzymology, a N6-hydroxylysine O-acetyltransferase (EC 2.3.1.102) is an enzyme that catalyzes the chemical reaction
In enzymology, an acetylglutamate kinase is an enzyme that catalyzes the chemical reaction:
In enzymology, a carbamate kinase (EC 2.7.2.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a streptomycin 6-kinase is an enzyme that catalyzes the chemical reaction

Aerobactin is a bacterial iron chelating agent (siderophore) found in E. coli. It is a virulence factor enabling E. coli to sequester iron in iron-poor environments such as the urinary tract.

In molecular biology, the citrate synthase family of proteins includes the enzymes citrate synthase EC 2.3.3.1, and the related enzymes 2-methylcitrate synthase EC 2.3.3.5 and ATP citrate lyase EC 2.3.3.8.

4-Hydroxy-tetrahydrodipicolinate synthase (EC 4.3.3.7, dihydrodipicolinate synthase, dihydropicolinate synthetase, dihydrodipicolinic acid synthase, L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing), dapA (gene)) is an enzyme with the systematic name L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing; (4S)-4-hydroxy-2,3,4,5-tetrahydro-(2S)-dipicolinate-forming). This enzyme catalyses the following chemical reaction







