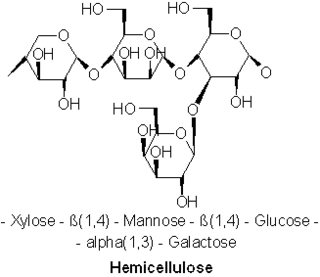
A hemicellulose is one of a number of heteropolymers, such as arabinoxylans, present along with cellulose in almost all terrestrial plant cell walls. Cellulose is crystalline, strong, and resistant to hydrolysis. Hemicelluloses are branched, shorter in length than cellulose, and also show a propensity to crystallize. They can be hydrolyzed by dilute acid or base as well as a myriad of hemicellulase enzymes.

Cellulase is any of several enzymes produced chiefly by fungi, bacteria, and protozoans that catalyze cellulolysis, the decomposition of cellulose and of some related polysaccharides:
Xyloglucan is a hemicellulose that occurs in the primary cell wall of all vascular plants; however, all enzymes responsible for xyloglucan metabolism are found in Charophyceae algae. In many dicotyledonous plants, it is the most abundant hemicellulose in the primary cell wall. Xyloglucan binds to the surface of cellulose microfibrils and may link them together. It is the substrate of xyloglucan endotransglycosylase, which cuts and ligates xyloglucans, as a means of integrating new xyloglucans into the cell wall. It is also thought to be the substrate of alpha-expansin, which promotes cell wall enlargement.

Brassinolide is a plant hormone. The first isolated brassinosteroid, it was discovered when it was shown that pollen from rapeseed could promote stem elongation and cell division. The biologically active component was isolated and named brassinolide.
Cellobiohydrolase may refer to:
The plant cell wall is made up of hydrated polymetric material, allowing it to have viscoelastic properties. The primary cell wall of a plant consists of cellulose fibers, hemicellulose, and xyloglucans. This load bearing network is also surrounded by pectins and glycoproteins.
In enzymology, a xyloglucan-specific endo-beta-1,4-glucanase (EC 3.2.1.151) is an enzyme that catalyzes the chemical reaction
In enzymology, a xyloglucan-specific exo-beta-1,4-glucanase (EC 3.2.1.155) is an enzyme that catalyzes the chemical reaction
In enzymology, a xyloglucan 4-glucosyltransferase is an enzyme that catalyzes the chemical reaction in which a beta-D-glucosyl residue is transferred from UDP-glucose to another glucose residue in xyloglucan, linked by a beta-1,4-D-glucosyl-D-glucose bond.
In enzymology, a xyloglucan 6-xylosyltransferase is an enzyme that catalyzes the chemical reaction in which an alpha-D-xylosyl residue is transferred from UDP-D-xylose to a glucose residue in xyloglucan, being attached by an alpha-1,6-D-xylosyl-D-glucose bond.
In enzymology, a xyloglucan:xyloglucosyl transferase (EC 2.4.1.207) is an enzyme that catalyzes the chemical reaction in which a beta-(1,4) bond in the backbone of a xyloglucan in broken; the xyloglucanyl segment is then transferred to the O4 of the non-reducing terminal glucose residue of either xyloglucan or an oligosaccharide thereof.
Mixed-linkage glucan : xyloglucan endotransglucosylase (MXE) is a plant cell wall-modifying enzyme found in plants of the genus Equisetum. The enzyme is proposed, in vivo, to catalyse the endotransglucosylation of two different hemicellulose polysaccharides, mixed-linkage glucan and xyloglucan, effectively 'stitching' them together. However only the 'stitching' of a mixed-linkage glucan polysaccharide to a xyloglucan oligosaccharide has actually been witnessed to date.

In molecular biology, glycoside hydrolase family 7 is a family of glycoside hydrolases EC 3.2.1., which are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycoside hydrolases, based on sequence similarity, has led to the definition of >100 different families. This classification is available on the CAZy web site, and also discussed at CAZypedia, an online encyclopedia of carbohydrate active enzymes.
In molecular biology, glycoside hydrolase family 6 is a family of glycoside hydrolases EC 3.2.1., which are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycoside hydrolases, based on sequence similarity, has led to the definition of >100 different families. This classification is available on the CAZy web site, and also discussed at CAZypedia, an online encyclopedia of carbohydrate active enzymes.

Glucanases are enzymes that break down large polysaccharides via hydrolysis. The product of the hydrolysis reaction is called a glucan, a linear polysaccharide made of up to 1200 glucose monomers, held together with glycosidic bonds. Glucans are abundant in the endosperm cell walls of cereals such as barley, rye, sorghum, rice, and wheat. Glucanases are also referred to as lichenases, hydrolases, glycosidases, glycosyl hydrolases, and/or laminarinases. Many types of glucanases share similar amino acid sequences but vastly different substrates. Of the known endo-glucanases, 1,3-1,4-β-glucanase is considered the most active.

In molecular biology, the xyloglucan endo-transglycosylase (XET) is an enzyme that is involved in the metabolism of xyloglucan, which is a component of plant cell walls. This enzyme is part of glycoside hydrolase family 16.
Cellulose 1,4-β-cellobiosidase is an enzyme of interest for its capability of converting cellulose to useful chemicals, particularly cellulosic ethanol.
Cellulose 1,4-β-cellobiosidase is an enzyme with systematic name 4-beta-D-glucan cellobiohydrolase . This enzyme catalyses the following chemical reaction
Alpha-D-xyloside xylohydrolase is an enzyme. This enzyme catalyses the following chemical reaction