
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group -NH
2 but is rather a secondary amine. The secondary amine nitrogen is in the protonated NH2+ form under biological conditions, while the carboxy group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG).
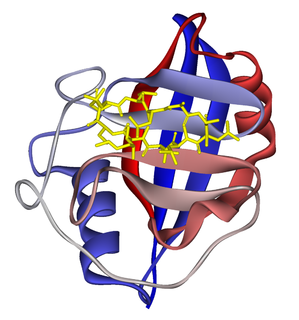
Cyclophilins (CYPs) are a family of proteins named after their ability to bind to ciclosporin, an immunosuppressant which is usually used to suppress rejection after internal organ transplants. They are found in all domains of life. These proteins have peptidyl prolyl isomerase activity, which catalyzes the isomerization of peptide bonds from trans form to cis form at proline residues and facilitates protein folding.
In molecular biology, immunophilins are endogenous cytosolic peptidyl-prolyl isomerases (PPI) that catalyze the interconversion between the cis and trans isomers of peptide bonds containing the amino acid proline (Pro). They are chaperon molecules that generally assist in the proper folding of diverse "client" proteins. Immunophilins are traditionally classified into two families that differ in sequence and biochemical characteristics. These two families are: "cyclosporin-binding cyclophilins (CyPs)" and "FK506-binding proteins (FKBPs)". Recently, a novel group of dual-family immunophilins (DFI) has been discovered, mostly in unicellular organisms; these DFIs are natural chimera of CyP and FKBPs, fused in either order.

Prolyl isomerase is an enzyme found in both prokaryotes and eukaryotes that interconverts the cis and trans isomers of peptide bonds with the amino acid proline. Proline has an unusually conformationally restrained peptide bond due to its cyclic structure with its side chain bonded to its secondary amine nitrogen. Most amino acids have a strong energetic preference for the trans peptide bond conformation due to steric hindrance, but proline's unusual structure stabilizes the cis form so that both isomers are populated under biologically relevant conditions. Proteins with prolyl isomerase activity include cyclophilin, FKBPs, and parvulin, although larger proteins can also contain prolyl isomerase domains.
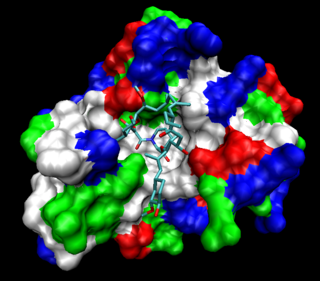
FKBP, or FK506 binding protein, is a family of proteins that have prolyl isomerase activity and are related to the cyclophilins in function, though not in amino acid sequence. FKBPs have been identified in many eukaryotes, ranging from yeast to humans, and function as protein folding chaperones for proteins containing proline residues. Along with cyclophilin, FKBPs belong to the immunophilin family.

Peptidylprolyl isomerase A (PPIA), also known as cyclophilin A (CypA) or rotamase A is an enzyme that in humans is encoded by the PPIA gene on chromosome 7. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to regulate many biological processes, including intracellular signaling, transcription, inflammation, and apoptosis. Due to its various functions, PPIA has been implicated in a broad range of inflammatory diseases, including atherosclerosis and arthritis, and viral infections.

Peptidyl-prolyl cis-trans isomerase FKBP1A is an enzyme that in humans is encoded by the FKBP1A gene.

Peptidyl-prolyl cis-trans isomerase B is an enzyme that is encoded by the PPIB gene. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to regulate protein folding of type I collagen. Generally, PPIases are found in all eubacteria and eukaryotes, as well as in a few archaebacteria, and thus are highly conserved.

Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 is an enzyme that in humans is encoded by the PIN1 gene.

Peptidylprolyl isomerase D , also known as PPID, is an enzyme which in humans is encoded by the PPID gene on chromosome 4. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to facilitate folding or repair of proteins. In addition, PPID participates in many biological processes, including mitochondrial metabolism, apoptosis, redox, and inflammation, as well as in related diseases and conditions, such as ischemic reperfusion injury, AIDS, and cancer.

Peptidyl-prolyl cis-trans isomerase, mitochondrial (PPIF) is an enzyme that in humans is encoded by the PPIF gene. It has also been referred to as, but should not be confused with, cyclophilin D (CypD), which is encoded by the PPID gene. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to facilitate folding or repair of proteins. PPIF is a major component of the mitochondrial permeability transition pore (MPTP) and, thus, highly involved in mitochondrial metabolism and apoptosis, as well as in mitochondrial diseases and related conditions, including cardiac diseases, neurodegenerative diseases, and muscular dystrophy. In addition, PPIF participates in inflammation, as well as in ischemic reperfusion injury, AIDS, and cancer.

Peptidyl-prolyl cis-trans isomerase NIMA-interacting 4 is an enzyme that in humans is encoded by the PIN4 gene.

Peptidyl-prolyl cis-trans isomerase-like 1 is an enzyme that in humans is encoded by the PPIL1 gene.

Peptidyl-prolyl cis-trans isomerase H is an enzyme that in humans is encoded by the PPIH gene.

Peptidyl-prolyl cis-trans isomerase C (PPIC) is an enzyme that in humans is encoded by the PPIC gene on chromosome 5. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to facilitate folding or repair of proteins. In addition, PPIC participates in many biological processes, including mitochondrial metabolism, apoptosis, redox, and inflammation, as well as in related diseases and conditions, such as ischemic reperfusion injury, AIDS, and cancer.

Peptidyl-prolyl cis-trans isomerase G is an enzyme that in humans is encoded by the PPIG gene.

Peptidylprolyl isomerase E , also known as PPIE, is an enzyme which in humans is encoded by the PPIE gene on chromosome 1. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to facilitate folding or repair of proteins. In addition, PPIE participates in many biological processes, including mitochondrial metabolism, apoptosis, and inflammation, as well as related diseases and conditions, such as ischemic reperfusion injury, AIDS, influenza, and cancer.
In epigenetics, proline isomerization is the effect that cis-trans isomerization of the amino acid proline has on the regulation of gene expression. Similar to aspartic acid, the amino acid proline has the rare property of being able to occupy both cis and trans isomers of its prolyl peptide bonds with ease. Peptidyl-prolyl isomerase, or PPIase, is an enzyme very commonly associated with proline isomerization due to their ability to catalyze the isomerization of prolines. PPIases are present in three types: cyclophilins, FK507-binding proteins, and the parvulins. PPIase enzymes catalyze the transition of proline between cis and trans isomers and are essential to the numerous biological functions controlled and affected by prolyl isomerization Without PPIases, prolyl peptide bonds will slowly switch between cis and trans isomers, a process that can lock proteins in a nonnative structure that can affect render the protein temporarily ineffective. Although this switch can occur on its own, PPIases are responsible for most isomerization of prolyl peptide bonds. The specific amino acid that precedes the prolyl peptide bond also can have an effect on which conformation the bond assumes. For instance, when an aromatic amino acid is bonded to a proline the bond is more favorable to the cis conformation. Cyclophilin A uses an "electrostatic handle" to pull proline into cis and trans formations. Most of these biological functions are affected by the isomerization of proline when one isomer interacts differently than the other, commonly causing an activation/deactivation relationship. As an amino acid, proline is present in many proteins. This aids in the multitude of effects that isomerization of proline can have in different biological mechanisms and functions.
Par14 is a member of the parvulin family of peptidyl-prolyl-cis/trans-isomerases (PPIases) in humans, which posseses prolyl isomerase activity.
Par17, a member of the parvulin family of peptidyl-prolyl-cis/trans-isomerases (PPIases), is an isoform of parvulin 14 (Par14) and originates from alternative transcription initiation of the PIN4 gene on chromosome Xq13 in humans. Par17, which carries a 25 residue N-terminal extension, shares the complete successive amino acid sequence with Par14. In contrast to Par14, Par17 is exclusively expressed in Hominidae, as ORFs were only found within the genomes of orangutan, gorilla, chimpanzee and humans. The N-terminal extension seems to be responsible for the translocation of Par17 to the mitochondrial matrix. Par17 is involved in tubulin and actin polymerization as well as in hepatitis B virus replication. Using photoaffinity labeling and liquid chromatography–mass spectrometry analysis, Par17 was found to be associated with mitochondrial proteins such as members of the electron transport chain in human cells.
















