DNA-binding proteins are proteins that have DNA-binding domains and thus have a specific or general affinity for single- or double-stranded DNA. Sequence-specific DNA-binding proteins generally interact with the major groove of B-DNA, because it exposes more functional groups that identify a base pair.
In molecular biology, immunophilins are endogenous cytosolic peptidyl-prolyl isomerases (PPI) that catalyze the interconversion between the cis and trans isomers of peptide bonds containing the amino acid proline (Pro). They are chaperone molecules that generally assist in the proper folding of diverse "client" proteins. Immunophilins are traditionally classified into two families that differ in sequence and biochemical characteristics. These two families are: "cyclosporin-binding cyclophilins (CyPs)" and "FK506-binding proteins (FKBPs)". In 2005, a group of dual-family immunophilins (DFI) has been discovered, mostly in unicellular organisms; these DFIs are natural chimera of CyP and FKBPs, fused in either order.
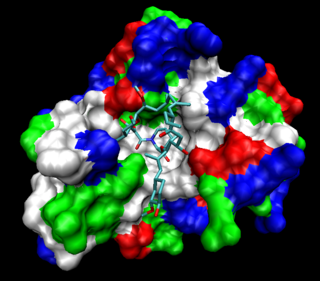
The FKBPs, or FK506 binding proteins, constitute a family of proteins that have prolyl isomerase activity and are related to the cyclophilins in function, though not in amino acid sequence. FKBPs have been identified in many eukaryotes, ranging from yeast to humans, and function as protein folding chaperones for proteins containing proline residues. Along with cyclophilin, FKBPs belong to the immunophilin family.

FK506-binding protein 4 is a protein that in humans is encoded by the FKBP4 gene.

Peptidyl-prolyl cis-trans isomerase FKBP1A is an enzyme that in humans is encoded by the FKBP1A gene. It is also commonly referred to as FKBP-12 or FKBP12 and is a member of a family of FK506-binding proteins (FKBPs).

Peptidyl-prolyl cis-trans isomerase B is an enzyme that is encoded by the PPIB gene. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to regulate protein folding of type I collagen. Generally, PPIases are found in all eubacteria and eukaryotes, as well as in a few archaebacteria, and thus are highly conserved.

Protein disulfide-isomerase, also known as the beta-subunit of prolyl 4-hydroxylase (P4HB), is an enzyme that in humans encoded by the P4HB gene. The human P4HB gene is localized in chromosome 17q25. Unlike other prolyl 4-hydroxylase family proteins, this protein is multifunctional and acts as an oxidoreductase for disulfide formation, breakage, and isomerization. The activity of P4HB is tightly regulated. Both dimer dissociation and substrate binding are likely to enhance its enzymatic activity during the catalysis process.

FK506 binding protein 5, also known as FKBP5, is a protein which in humans is encoded by the FKBP5 gene.

FK506-binding protein 8 is a protein that in humans is encoded by the FKBP8 gene.
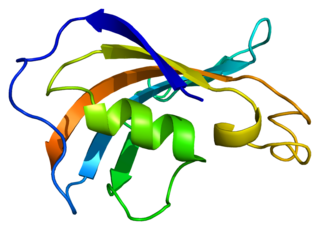
Peptidyl-prolyl cis-trans isomerase FKBP1B is an enzyme that in humans is encoded by the FKBP1B gene.

Peptidylprolyl isomerase D (cyclophilin D), also known as PPID, is an enzyme which in humans is encoded by the PPID gene on chromosome 4. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to facilitate folding or repair of proteins. In addition, PPID participates in many biological processes, including mitochondrial metabolism, apoptosis, redox, and inflammation, as well as in related diseases and conditions, such as ischemic reperfusion injury, AIDS, and cancer.

Glomulin is a protein that in humans is encoded by the GLMN gene.

FK506-binding protein 2 is a protein that in humans is encoded by the FKBP2 gene.

Peptidyl-prolyl cis-trans isomerase NIMA-interacting 4 is an enzyme that in humans is encoded by the PIN4 gene.

Peptidyl-prolyl cis-trans isomerase H is an enzyme that in humans is encoded by the PPIH gene.

Peptidyl-prolyl cis-trans isomerase C (PPIC) is an enzyme that in humans is encoded by the PPIC gene on chromosome 5. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to facilitate folding or repair of proteins. In addition, PPIC participates in many biological processes, including mitochondrial metabolism, apoptosis, redox, and inflammation, as well as in related diseases and conditions, such as ischemic reperfusion injury, AIDS, and cancer.

Peptidyl-prolyl cis-trans isomerase G is an enzyme that in humans is encoded by the PPIG gene.

Peptidylprolyl isomerase E (cyclophilin E), also known as PPIE, is an enzyme which in humans is encoded by the PPIE gene on chromosome 1. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to facilitate folding or repair of proteins. In addition, PPIE participates in many biological processes, including mitochondrial metabolism, apoptosis, and inflammation, as well as related diseases and conditions, such as ischemic reperfusion injury, AIDS, influenza, and cancer.

FK506-binding protein 10 is a protein that in humans is encoded by the FKBP10 gene.

FK506 binding protein 6, also known as FKBP6, is a human gene. The encoded protein shows structural homology to FKBP immunophilins, which bind to the immunosuppressants FK506 and rapamycin.