Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Food are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.

Peroxidases or peroxide reductases are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides.

Glutathione peroxidase (GPx) is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid hydroperoxides to their corresponding alcohols and to reduce free hydrogen peroxide to water.
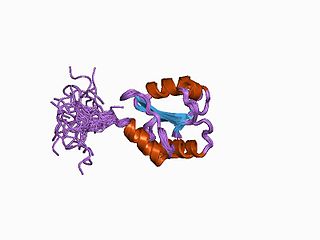
Protein disulfide isomerase, or PDI, is an enzyme in the endoplasmic reticulum (ER) in eukaryotes and the periplasm of bacteria that catalyzes the formation and breakage of disulfide bonds between cysteine residues within proteins as they fold. This allows proteins to quickly find the correct arrangement of disulfide bonds in their fully folded state, and therefore the enzyme acts to catalyze protein folding.

Lipid peroxidation is the chain of reactions of oxidative degradation of lipids. It is the process in which free radicals "steal" electrons from the lipids in cell membranes, resulting in cell damage. This process proceeds by a free radical chain reaction mechanism. It most often affects polyunsaturated fatty acids, because they contain multiple double bonds in between which lie methylene bridges (-CH2-) that possess especially reactive hydrogen atoms. As with any radical reaction, the reaction consists of three major steps: initiation, propagation, and termination. The chemical products of this oxidation are known as lipid peroxides or lipid oxidation products (LOPs).

Glutathione disulfide (GSSG) is a disulfide derived from two glutathione molecules.

Sulfur assimilation is the process by which living organisms incorporate sulfur into their biological molecules. In plants, sulfate is absorbed by the roots and then be transported to the chloroplasts by the transipration stream where the sulfur are reduced to sulfide with the help of a series of enzymatic reactions. Furthermore, the reduced sulfur is incorporated into cysteine, an amino acid that is a precursor to many other sulfur-containing compounds. In animals, sulfur assimilation occurs primarily through the diet, as animals cannot produce sulfur-containing compounds directly. Sulfur is incorporated into amino acids such as cysteine and methionine, which are used to build proteins and other important molecules. Besides, With the rapid development of economy, the increase emission of sulfur results in environmental issues, such as acid rain and hydrogen sulfilde.
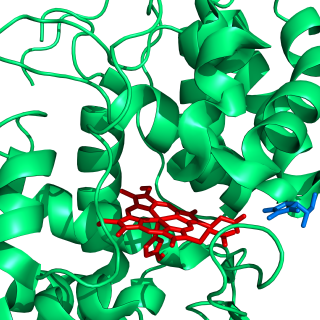
Ascorbate peroxidase (or L-ascorbate peroxidase, APX) (EC 1.11.1.11) is an enzyme that catalyzes the chemical reaction
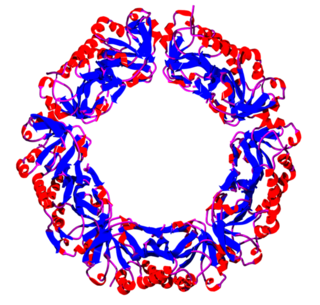
Peroxiredoxins are a ubiquitous family of antioxidant enzymes that also control cytokine-induced peroxide levels and thereby mediate signal transduction in mammalian cells. The family members in humans are PRDX1, PRDX2, PRDX3, PRDX4, PRDX5, and PRDX6. The physiological importance of peroxiredoxins is indicated by their relative abundance. Their function is the reduction of peroxides, specifically hydrogen peroxide, alkyl hydroperoxides, and peroxynitrite.
In enzymology, a methylarsonate reductase (EC 1.20.4.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a fatty-acid peroxidase (EC 1.11.1.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a manganese peroxidase (EC 1.11.1.13) is an enzyme that catalyzes the chemical reaction

In enzymology, a NADH peroxidase (EC 1.11.1.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a NADPH peroxidase (EC 1.11.1.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a protein-disulfide reductase (glutathione) is an enzyme that catalyzes the chemical reaction
In enzymology, a pyrimidodiazepine synthase (EC 1.5.4.1) is an enzyme that catalyzes the chemical reaction

Glutathione peroxidase 4, also known as GPX4, is an enzyme that in humans is encoded by the GPX4 gene. GPX4 is a phospholipid hydroperoxidase that protects cells against membrane lipid peroxidation.
Glutathione amide-dependent peroxidase (EC 1.11.1.17) is an enzyme with systematic name glutathione amide:hydrogen-peroxide oxidoreductase. This enzyme catalyses the following chemical reaction
Oxidation response is stimulated by a disturbance in the balance between the production of reactive oxygen species and antioxidant responses, known as oxidative stress. Active species of oxygen naturally occur in aerobic cells and have both intracellular and extracellular sources. These species, if not controlled, damage all components of the cell, including proteins, lipids and DNA. Hence cells need to maintain a strong defense against the damage. The following table gives an idea of the antioxidant defense system in bacterial system.

Bis(trifluoromethyl)peroxide (BTP) is a fluorocarbon derivative first produced by Frédéric Swarts. It has some utility as a radical initiator for polymerisation reactions. BTP is unusual in the fact that, unlike many peroxides, it is a gas, is non-explosive, and has good thermal stability.










