| |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral, intramuscular injection |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.375 |
| Chemical and physical data | |
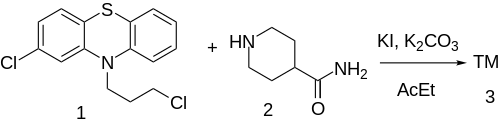
| Formula | C21H24ClN3OS |
| Molar mass | 401.95 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Pipamazine (INN; trade names Mornidine, Mometine, Nausidol) is a drug of the phenothiazine class formerly used as an antiemetic. It is chemically related to chlorpromazine, but has negligible antipsychotic activity and produces few extrapyramidal side effects. [1]
Contents
Pipamazine was introduced to the U.S. market in 1959 by G. D. Searle & Company. It was advertised for morning sickness [2] and postoperative nausea and vomiting, and was claimed to reduce the need for postoperative analgesia. [3] It was eventually withdrawn from the U.S. market in 1969, after reports of hepatotoxicity (liver injury). [4] [5]
There is very little published information on pipamazine; it is mostly absent from modern-day sources, apart from a few passing mentions in the pharmacological literature. [1]