
Methionine synthase also known as MS, MeSe, MTR is responsible for the regeneration of methionine from homocysteine. In humans it is encoded by the MTR gene (5-methyltetrahydrofolate-homocysteine methyltransferase). Methionine synthase forms part of the S-adenosylmethionine (SAMe) biosynthesis and regeneration cycle, and is the enzyme responsible for linking the cycle to one-carbon metabolism via the folate cycle. There are two primary forms of this enzyme, the Vitamin B12 (cobalamin)-dependent (MetH) and independent (MetE) forms, although minimal core methionine synthases that do not fit cleanly into either category have also been described in some anaerobic bacteria. The two dominant forms of the enzymes appear to be evolutionary independent and rely on considerably different chemical mechanisms. Mammals and other higher eukaryotes express only the cobalamin-dependent form. In contrast, the distribution of the two forms in Archaeplastida (plants and algae) is more complex. Plants exclusively possess the cobalamin-independent form, while algae have either one of the two, depending on species. Many different microorganisms express both the cobalamin-dependent and cobalamin-independent forms.
In enzymology, a cobalt-factor II C20-methyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a magnesium protoporphyrin IX methyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a precorrin-2 C20-methyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, precorrin-3B C17-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a precorrin-4 C11-methyltransferase is an enzyme that catalyzes the chemical reaction
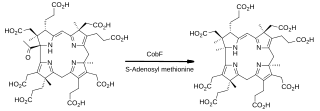
In enzymology, precorrin-6A synthase (deacetylating) (EC 2.1.1.152) is an enzyme that catalyzes the chemical reaction

In enzymology, a precorrin-2 dehydrogenase (EC 1.3.1.76) is an enzyme that catalyzes the chemical reaction
In enzymology, a precorrin-6A reductase (EC 1.3.1.54) is an enzyme that catalyzes the chemical reaction
In enzymology, a precorrin-3B synthase (EC 1.14.13.83) is an enzyme that catalyzes the chemical reaction

In enzymology, a cob(II)yrinic acid a,c-diamide reductase is an enzyme that catalyzes the chemical reaction

[Methionine synthase] reductase, or Methionine synthase reductase, encoded by the gene MTRR, is an enzyme that is responsible for the reduction of methionine synthase inside human body. This enzyme is crucial for maintaining the one carbon metabolism, specifically the folate cycle. The enzyme employs one coenzyme, flavoprotein.

In enzymology, a precorrin-8X methylmutase is an enzyme that catalyzes the chemical reaction
The enzyme threonine-phosphate decarboxylase (EC 4.1.1.81) catalyzes the chemical reaction

Cobalt chelatase (EC 6.6.1.2) is an enzyme that catalyzes the chemical reaction

In enzymology, a nicotinate-nucleotide-dimethylbenzimidazole phosphoribosyltransferase is an enzyme that catalyzes the chemical reaction

Cobalamin biosynthesis is the process by which bacteria and archea make cobalamin, vitamin B12. Many steps are involved in converting aminolevulinic acid via uroporphyrinogen III and adenosylcobyric acid to the final forms in which it is used by enzymes in both the producing organisms and other species, including humans who acquire it through their diet.

Uroporphyrinogen-III C-methyltransferase, uroporphyrinogen methyltransferase, uroporphyrinogen-III methyltransferase, adenosylmethionine-uroporphyrinogen III methyltransferase, S-adenosyl-L-methionine-dependent uroporphyrinogen III methylase, uroporphyrinogen-III methylase, SirA, CysG, CobA, uroporphyrin-III C-methyltransferase, S-adenosyl-L-methionine:uroporphyrin-III C-methyltransferase) is an enzyme with systematic name S-adenosyl-L-methionine:uroporphyrinogen-III C-methyltransferase. This enzyme catalyses the following chemical reaction
Cobalt-precorrin-5B (C1)-methyltransferase (EC 2.1.1.195), cobalt-precorrin-6A synthase, CbiD (gene)) is an enzyme with systematic name S-adenosyl-L-methionine:cobalt-precorrin-5B (C1)-methyltransferase. This enzyme catalyses the following chemical reaction
Cobalt-precorrin-7 (C15)-methyltransferase (decarboxylating) (EC 2.1.1.196, CbiT) is an enzyme with systematic name S-adenosyl-L-methionine:precorrin-7 C15-methyltransferase (C12-decarboxylating). This enzyme catalyses the following chemical reaction














