
Pythium is a genus of parasitic oomycetes. They were formerly classified as fungi. Most species are plant parasites, but Pythium insidiosum is an important pathogen of animals, causing pythiosis. The feet of the fungus gnat are frequently a vector for their transmission.
Phytophthora sojae is an oomycete and a soil-borne plant pathogen that causes stem and root rot of soybean. This is a prevalent disease in most soybean growing regions, and a major cause of crop loss. In wet conditions the pathogen produces zoospores that move in water and are attracted to soybean roots. Zoospores can attach to roots, germinate, and infect the plant tissues. Diseased roots develop lesions that may spread up the stem and eventually kill the entire plant. Phytophthora sojae also produces oospores that can remain dormant in the soil over the winter, or longer, and germinate when conditions are favourable. Oospores may also be spread by animals or machinery.

Phytophthora palmivora is an oomycete that causes bud-rot of palms, fruit-rot or kole-roga of coconut and areca nut. These are among the most serious diseases caused by fungi and moulds in South India. It occurs almost every year in Malnad, Mysore, North & South Kanara, Malabar and other areas. Similar diseases of palms are also known to occur in Sri Lanka, Mauritius, and Sumatra. The causative organism was first identified as P. palmivora by Edwin John Butler in 1917.
Aphanomyces euteiches is a water mould, or oomycete, plant pathogen responsible for the disease Aphanomyces root rot. The species Aphanomyces euteiches can infect a variety of legumes. Symptoms of the disease can differ among hosts but generally include reduced root volume and function, leading to stunting and chlorotic foliage. Aphanomyces root rot is an important agricultural disease in the United States, Europe, Australia, New Zealand, and Japan. Management includes using resistant crop varieties and having good soil drainage, as well as testing soil for the pathogen to avoid infected fields.
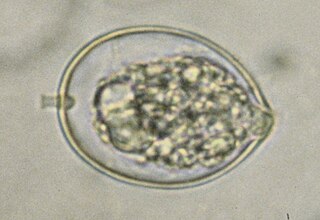
Phytophthora cactorum is a fungal-like plant pathogen belonging to the Oomycota phylum. It is the causal agent of root rot on rhododendron and many other species, as well as leather rot of strawberries.

Phytophthora medicaginis is an oomycete plant pathogen that causes root rot in alfalfa and chickpea. It is a major disease of these plants and is found wherever they are grown. P. medicaginis causes failure of stand establishment because of seedling death. Phytophthora medicaginis is part of a species complex with Phytophthora megasperma.
Phytophthora nicotianae or black shank is an oomycete belonging to the order Peronosporales and family Peronosporaceae.
Pythium irregulare is a soil borne oomycete plant pathogen. Oomycetes, also known as "water molds", are fungal-like protists. They are fungal-like because of their similar life cycles, but differ in that the resting stage is diploid, they have coenocytic hyphae, a larger genome, cellulose in their cell walls instead of chitin, and contain zoospores and oospores.
Pythium ultimum is a plant pathogen. It causes damping off and root rot diseases of hundreds of diverse plant hosts including corn, soybean, potato, wheat, fir, and many ornamental species. P. ultimum belongs to the peronosporalean lineage of oomycetes, along with other important plant pathogens such as Phytophthora spp. and many genera of downy mildews. P. ultimum is a frequent inhabitant of fields, freshwater ponds, and decomposing vegetation in most areas of the world. Contributing to the widespread distribution and persistence of P. ultimum is its ability to grow saprotrophically in soil and plant residue. This trait is also exhibited by most Pythium spp. but not by the related Phytophthora spp., which can only colonize living plant hosts.

Phytophthora erythroseptica—also known as pink rot along with several other species of Phytophthora—is a plant pathogen. It infects potatoes causing their tubers to turn pink and damages leaves. It also infects tulips (Tulipa) damaging their leaves and shoots.
Pythium aphanidermatum is a soil borne plant pathogen. Pythium is a genus in the class Oomycetes, which are also known as water molds. Oomycetes are not true fungi, as their cell walls are made of cellulose instead of chitin, they are diploid in their vegetative state, and they form coenocytic hyphae. Also, they reproduce asexually with motile biflagelette zoospores that require water to move towards and infect a host. Sexually, they reproduce with structures called antheridia, oogonia, and oospores.
Pythium graminicola is a plant pathogen infecting cereals.
Pythium myriotylum is a soil-borne oomycete necrotroph that has a broad host range, this means that it can infect a wide range of plants.
Pythium volutum is a plant pathogen infecting wheat, barley, and turfgrass. It is known to be sensitive to some of the compounds typically present in selective media commonly used for isolating Pythium spp., so isolation may require alternative methods.
Aphanomyces cochlioides is a plant pathogen that can affect commodity crops like spinach, Swiss chard, beets and related species. In spinach the pathogen is responsible for the black root "rot" that can damage plants.

Phytophthora capsici is an oomycete plant pathogen that causes blight and fruit rot of peppers and other important commercial crops. It was first described by L. Leonian at the New Mexico State University Agricultural Experiment Station in Las Cruces in 1922 on a crop of chili peppers. In 1967, a study by M. M. Satour and E. E. Butler found 45 species of cultivated plants and weeds susceptible to P. capsici In Greek, Phytophthora capsici means "plant destroyer of capsicums". P. capsici has a wide range of hosts including members of the families Solanaceae and Cucurbitaceae as well as Fabaceae.
Pythium sulcatum is a chromalveolate plant pathogen infecting carrots. Because this organism was once thought to be a type of fungus, it is still often treated as such.
Globisporangium sylvaticum is a plant pathogen, an oomycete known to cause root rot and damping off in a multitude of species. These species include apples, carrot, cherry laurel, cress, cucumber, garlic, lettuce, pea, rhododendron, and spinach. Symptoms of infection include stunting, wilt, chlorosis, and browning and eventual necrosis of roots. The pathogen can by identified by the presence of thick, microscopic, round spores within the cells of the root.
Phytophthora hydropathica is an oomycete plant pathogen that is found in aquatic environments such as irrigation and river water. The pathogen was previously classified as P. drechsleri Dre II before being categorized as its own distinct species. P. hydropathica has been primarily found in association with ornamental plant nurseries. The pathogen has been isolated throughout the Southern United States, as well as internationally in Mexico, Italy, and Spain.
Black rot on orchids is caused by Pythium and Phytophthora species. Black rot targets a variety of orchids but Cattleya orchids are especially susceptible. Pythium ultimum and Phytophthora cactorum are known to cause black rot in orchids.






