
Cytochrome P450 3A4 is an important enzyme in the body, mainly found in the liver and in the intestine, which in humans is encoded by CYP3A4 gene. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme.

Cytochrome P450 2E1 is a member of the cytochrome P450 mixed-function oxidase system, which is involved in the metabolism of xenobiotics in the body. This class of enzymes is divided up into a number of subcategories, including CYP1, CYP2, and CYP3, which as a group are largely responsible for the breakdown of foreign compounds in mammals.

Aromatase, also called estrogen synthetase or estrogen synthase, is an enzyme responsible for a key step in the biosynthesis of estrogens. It is CYP19A1, a member of the cytochrome P450 superfamily, which are monooxygenases that catalyze many reactions involved in steroidogenesis. In particular, aromatase is responsible for the aromatization of androgens into estrogens. The enzyme aromatase can be found in many tissues including gonads, brain, adipose tissue, placenta, blood vessels, skin, and bone, as well as in tissue of endometriosis, uterine fibroids, breast cancer, and endometrial cancer. It is an important factor in sexual development.

Gingerol ([6]-gingerol) is a phenolic phytochemical compound found in fresh ginger that activates heat receptors on the tongue. It is normally found as a pungent yellow oil in the ginger rhizome, but can also form a low-melting crystalline solid. This chemical compound is found in all members of the Zingiberaceae family and is high in concentrations in the grains of paradise as well as an African Ginger species.

Cytochrome P450, family 1, subfamily A, polypeptide 1 is a protein that in humans is encoded by the CYP1A1 gene. The protein is a member of the cytochrome P450 superfamily of enzymes.

Pinosylvin is an organic compound with the formula C6H5CH=CHC6H3(OH)2. A white solid, it is related to trans-stilbene, but with two hydroxy groups on one of the phenyl substituents. It is very soluble in many organic solvents, such as acetone.

Piceatannol is the organic compound with the formula ( 2C6H3)2CH)2. Classified as a stilbenoid and a phenol, it is a white solid, although samples often are yellow owing to impurities.

Cytochrome P450 3A5 is a protein that in humans is encoded by the CYP3A5 gene.

Cytochrome P450 2J2 (CYP2J2) is a protein that in humans is encoded by the CYP2J2 gene. CYP2J2 is a member of the cytochrome P450 superfamily of enzymes. The enzymes are oxygenases which catalyze many reactions involved in the metabolism of drugs and other xenobiotics) as well as in the synthesis of cholesterol, steroids and other lipids.

Cytochrome P450 4A11 is a protein that in humans is codified by the CYP4A11 gene.

Cytochrome P450 26A1 is a protein that in humans is encoded by the CYP26A1 gene.

25-hydroxycholesterol 7-alpha-hydroxylase also known as oxysterol and steroid 7-alpha-hydroxylase is an enzyme that in humans is encoded by the CYP7B1 gene. This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids.

Cytochrome P450 4F8 is a protein that in humans is encoded by the CYP4F8 gene.

Cytochrome P450 4F3, also leukotriene-B(4) omega-hydroxylase 2, is an enzyme that in humans is encoded by the CYP4F3 gene. CYP4F3 encodes two distinct enzymes, CYP4F3A and CYP4F3B, which originate from the alternative splicing of a single pre-mRNA precursor molecule; selection of either isoform is tissue-specific with CYP3F3A being expressed mostly in leukocytes and CYP4F3B mostly in the liver.

Vitis coignetiae, called crimson glory vine, is a plant belonging to the genus Vitis that is native to the temperate climes of Asia, where it can be found in the Russian Far East, (Sakhalin); Korea; and Japan. It was described botanically in 1883. It is called meoru (머루) in Korean and yama-budo (ヤマブドウ) in Japanese.
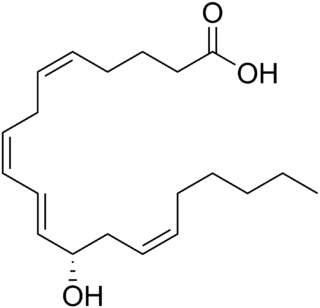
12-Hydroxyeicosatetraenoic acid (12-HETE) is a derivative of the 20 carbon polyunsaturated fatty acid, arachidonic acid, containing a hydroxyl residue at carbon 12 and a 5Z,8Z,10E,14Z Cis–trans isomerism configuration (Z=cis, E=trans) in its four double bonds. It was first found as a product of arachidonic acid metabolism made by human and bovine platelets through their 12S-lipoxygenase (i.e. ALOX12) enzyme(s). However, the term 12-HETE is ambiguous in that it has been used to indicate not only the initially detected "S" stereoisomer, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HETE or 12S-HETE), made by platelets, but also the later detected "R" stereoisomer, 12(R)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (also termed 12(R)-HETE or 12R-HETE) made by other tissues through their 12R-lipoxygenase enzyme, ALOX12B. The two isomers, either directly or after being further metabolized, have been suggested to be involved in a variety of human physiological and pathological reactions. Unlike hormones which are secreted by cells, travel in the circulation to alter the behavior of distant cells, and thereby act as Endocrine signalling agents, these arachidonic acid metabolites act locally as Autocrine signalling and/or Paracrine signaling agents to regulate the behavior of their cells of origin or of nearby cells, respectively. In these roles, they may amplify or dampen, expand or contract cellular and tissue responses to disturbances.

Isorhapontigenin is a tetrahydroxylated stilbenoid with a methoxy group. It is an isomer of rhapontigenin and an analog of resveratrol. It is found in the Chinese herb Gnetum cleistostachyum, in Gnetum parvifolium and in the seeds of the palm Aiphanes aculeata.
Gnetum cleistostachyum is a liana species in the Sessiles subsection of the genus Gnetum described from South East Yunnan.
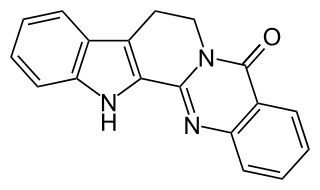
Rutecarpine or rutaecarpine is a COX-2 inhibitor isolated from Tetradium ruticarpum, a tree native to China. It is classified as a non-basic alkaloid.

20-Hydroxyeicosatetraenoic acid, also known as 20-HETE or 20-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid, is an eicosanoid metabolite of arachidonic acid that has a wide range of effects on the vascular system including the regulation of vascular tone, blood flow to specific organs, sodium and fluid transport in the kidney, and vascular pathway remodeling. These vascular and kidney effects of 20-HETE have been shown to be responsible for regulating blood pressure and blood flow to specific organs in rodents; genetic and preclinical studies suggest that 20-HETE may similarly regulate blood pressure and contribute to the development of stroke and heart attacks. Additionally the loss of its production appears to be one cause of the human neurological disease, Hereditary spastic paraplegia. Preclinical studies also suggest that the overproduction of 20-HETE may contribute to the progression of certain human cancers, particularly those of the breast.


















