
In organic chemistry, a diene ; also diolefin, dy-OH-lə-fin) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alkene units, with the standard prefix di of systematic nomenclature. As a subunit of more complex molecules, dienes occur in naturally occurring and synthetic chemicals and are used in organic synthesis. Conjugated dienes are widely used as monomers in the polymer industry. Polyunsaturated fats are of interest to nutrition.
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2.

Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species along with 1,2-diselenolene derivatives, are unsaturated bidentate ligand wherein the two donor atoms are sulfur. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.

In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.
Bis(oxazoline) ligands (often abbreviated BOX ligands) are a class of privileged chiral ligands containing two oxazoline rings. They are typically C2‑symmetric and exist in a wide variety of forms; with structures based around CH2 or pyridine linkers being particularly common (often generalised BOX and PyBOX respectively). The coordination complexes of bis(oxazoline) ligands are used in asymmetric catalysis. These ligands are examples of C2-symmetric ligands.

Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts.

Salcomine is a coordination complex derived from the salen ligand and cobalt. The complex, which is planar, and a variety of its derivatives are carriers for O2 as well as oxidation catalysts.

Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines, which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond.

Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a coordination compound of manganese and a salen-type ligand. It is used as an asymmetric catalyst in the Jacobsen epoxidation, which is renowned for its ability to enantioselectively transform prochiral alkenes into epoxides. Before its development, catalysts for the asymmetric epoxidation of alkenes required the substrate to have a directing functional group, such as an alcohol as seen in the Sharpless epoxidation. This compound has two enantiomers, which give the appropriate epoxide product from the alkene starting material.

Germylenes are a class of germanium(II) compounds with the general formula :GeR2. They are heavier carbene analogs. However, unlike carbenes, whose ground state can be either singlet or triplet depending on the substituents, germylenes have exclusively a singlet ground state. Unprotected carbene analogs, including germylenes, has a dimerization nature. Free germylenes can be isolated under the stabilization of steric hindrance or electron donation. The synthesis of first stable free dialkyl germylene was reported by Jutzi, et al in 1991.
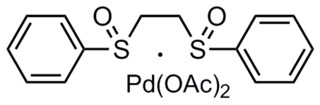
The White catalyst is a transition metal coordination complex named after the chemist by whom it was first synthesized, M. Christina White, a professor at the University of Illinois. The catalyst has been used in a variety of allylic C-H functionalization reactions of α-olefins. In addition, it has been shown to catalyze oxidative Heck reactions.

A metal salen complex is a coordination compound between a metal cation and a ligand derived from N,N′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent cobalt Co2+, usually denoted as Co(salen). These complexes are widely investigated as catalysts and enzyme mimics.

1,2-Diaminopropane (propane-1,2-diamine) is organic compound with the formula CH3CH(NH2)CH2NH2. A colorless liquid, it is the simplest chiral diamine. It is used as a bidentate ligand in coordination chemistry.
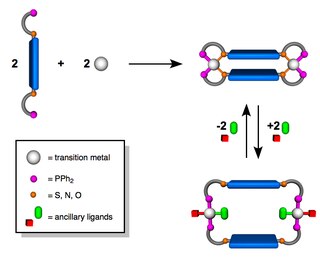
The Weak-Link Approach (WLA) is a supramolecular coordination-based assembly methodology, first introduced in 1998 by the Mirkin Group at Northwestern University. This method takes advantage of hemilabile ligands -ligands that contain both strong and weak binding moieties- that can coordinate to metal centers and quantitatively assemble into a single condensed ‘closed’ structure. Unlike other supramolecular assembly methods, the WLA allows for the synthesis of supramolecular complexes that can be modulated from rigid ‘closed’ structures to flexible ‘open’ structures through reversible binding of allosteric effectors at the structural metal centers. The approach is general and has been applied to a variety of metal centers and ligand designs including those with utility in catalysis and allosteric regulation.
Diimines are organic compounds containing two imine (RCH=NR') groups. Common derivatives are 1,2-diimines and 1,3-diimines. These compounds are used as ligands, but they are also precursors to other organic compounds.
In organometallic chemistry, a transition metal alkyne complex is a coordination compound containing one or more alkyne ligands. Such compounds are intermediates in many catalytic reactions that convert alkynes to other organic products, e.g. hydrogenation and trimerization.

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.

1,5-Diaza-3,7-diphosphacyclooctanes are organophosphorus compounds with the formula [R'NCH2P(R)CH2]2, often abbreviated PR2NR'2. They are air-sensitive white solids that are soluble in organic solvents. The ligands exist as meso and d,l-diastereomers, but only the meso forms function as bidentate ligands.
In homogeneous catalysis, C2-symmetric ligands refer to ligands that lack mirror symmetry but have C2 symmetry. Such ligands are usually bidentate and are valuable in catalysis. The C2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. C2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including C2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product.
Transition metal amino acid complexes are a large family of coordination complexes containing the conjugate bases of the amino acids, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the transition metals. Not included in this article are complexes of the amides and ester derivatives of amino acids. Also excluded are the polyamino acids including the chelating agents EDTA and NTA.


















