
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in Earth's crust. Selenium was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.
Primary nutritional groups are groups of organisms, divided in relation to the nutrition mode according to the sources of energy and carbon, needed for living, growth and reproduction. The sources of energy can be light or chemical compounds; the sources of carbon can be of organic or inorganic origin.

A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the carbon cycle, the nitrogen cycle and the water cycle. In each cycle, the chemical element or molecule is transformed and cycled by living organisms and through various geological forms and reservoirs, including the atmosphere, the soil and the oceans. It can be thought of as the pathway by which a chemical substance cycles the biotic compartment and the abiotic compartments of Earth. The biotic compartment is the biosphere and the abiotic compartments are the atmosphere, lithosphere and hydrosphere.

Bioremediation broadly refers to any process wherein a biological system, living or dead, is employed for removing environmental pollutants from air, water, soil, flue gasses, industrial effluents etc., in natural or artificial settings. The natural ability of organisms to adsorb, accumulate, and degrade common and emerging pollutants has attracted the use of biological resources in treatment of contaminated environment. In comparison to conventional physicochemical treatment methods bioremediation may offer considerable advantages as it aims to be sustainable, eco-friendly, cheap, and scalable. Most bioremediation is inadvertent, involving native organisms. Research on bioremediation is heavily focused on stimulating the process by inoculation of a polluted site with organisms or supplying nutrients to promote the growth. In principle, bioremediation could be used to reduce the impact of byproducts created from anthropogenic activities, such as industrialization and agricultural processes. Bioremediation could prove less expensive and more sustainable than other remediation alternatives.
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.
The pedosphere is the outermost layer of the Earth that is composed of soil and subject to soil formation processes. It exists at the interface of the lithosphere, atmosphere, hydrosphere and biosphere. The pedosphere is the skin of the Earth and only develops when there is a dynamic interaction between the atmosphere, biosphere, lithosphere and the hydrosphere. The pedosphere is the foundation of terrestrial life on Earth.

Phototrophs are organisms that carry out photon capture to produce complex organic compounds and acquire energy. They use the energy from light to carry out various cellular metabolic processes. It is a common misconception that phototrophs are obligatorily photosynthetic. Many, but not all, phototrophs often photosynthesize: they anabolically convert carbon dioxide into organic material to be utilized structurally, functionally, or as a source for later catabolic processes. All phototrophs either use electron transport chains or direct proton pumping to establish an electrochemical gradient which is utilized by ATP synthase, to provide the molecular energy currency for the cell. Phototrophs can be either autotrophs or heterotrophs. If their electron and hydrogen donors are inorganic compounds they can be also called lithotrophs, and so, some photoautotrophs are also called photolithoautotrophs. Examples of phototroph organisms are Rhodobacter capsulatus, Chromatium, and Chlorobium.

Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate (SO2−
4) as terminal electron acceptor, reducing it to hydrogen sulfide (H2S). Therefore, these sulfidogenic microorganisms "breathe" sulfate rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration.

In biology, detritus is dead particulate organic material, as distinguished from dissolved organic material. Detritus typically includes the bodies or fragments of bodies of dead organisms, and fecal material. Detritus typically hosts communities of microorganisms that colonize and decompose it. In terrestrial ecosystems it is present as leaf litter and other organic matter that is intermixed with soil, which is denominated "soil organic matter". The detritus of aquatic ecosystems is organic material that is suspended in the water and accumulates in depositions on the floor of the body of water; when this floor is a seabed, such a deposition is denominated "marine snow".
Lithotrophs are a diverse group of organisms using an inorganic substrate to obtain reducing equivalents for use in biosynthesis or energy conservation via aerobic or anaerobic respiration. While lithotrophs in the broader sense include photolithotrophs like plants, chemolithotrophs are exclusively microorganisms; no known macrofauna possesses the ability to use inorganic compounds as electron sources. Macrofauna and lithotrophs can form symbiotic relationships, in which case the lithotrophs are called "prokaryotic symbionts". An example of this is chemolithotrophic bacteria in giant tube worms or plastids, which are organelles within plant cells that may have evolved from photolithotrophic cyanobacteria-like organisms. Chemolithotrophs belong to the domains Bacteria and Archaea. The term "lithotroph" was created from the Greek terms 'lithos' (rock) and 'troph' (consumer), meaning "eaters of rock". Many but not all lithoautotrophs are extremophiles.
In biogeochemistry, remineralisation refers to the breakdown or transformation of organic matter into its simplest inorganic forms. These transformations form a crucial link within ecosystems as they are responsible for liberating the energy stored in organic molecules and recycling matter within the system to be reused as nutrients by other organisms.
Soil chemistry is the study of the chemical characteristics of soil. Soil chemistry is affected by mineral composition, organic matter and environmental factors. In the early 1870s a consulting chemist to the Royal Agricultural Society in England, named J. Thomas Way, performed many experiments on how soils exchange ions, and is considered the father of soil chemistry. Other scientists who contributed to this branch of ecology include Edmund Ruffin, and Linus Pauling.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.

The phosphorus cycle is the biogeochemical cycle that describes the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the movement of phosphorus, because phosphorus and phosphorus-based compounds are usually solids at the typical ranges of temperature and pressure found on Earth. The production of phosphine gas occurs in only specialized, local conditions. Therefore, the phosphorus cycle should be viewed from whole Earth system and then specifically focused on the cycle in terrestrial and aquatic systems.
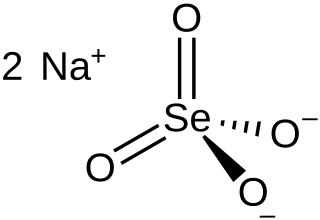
Sodium selenate is the inorganic compound with the formula Na
2SeO
4, not to be confused with sodium selenite. It exists as the anhydrous salt, the heptahydrate, and the decahydrate. These are white, water-soluble solids. The decahydrate is a common ingredient in multivitamins and livestock feed as a source of selenium. The anhydrous salt is used in the production of some glass. Although the selenates are much more toxic, many physical properties of sodium selenate and sodium sulfate are similar.
Sulfur is metabolized by all organisms, from bacteria and archaea to plants and animals. Sulfur can have an oxidation state from -2 to +6 and is reduced or oxidized by a diverse range of organisms. The element is present in proteins, sulfate esters of polysaccharides, steroids, phenols, and sulfur-containing coenzymes.

A redox gradient is a series of reduction-oxidation (redox) reactions sorted according to redox potential. The redox ladder displays the order in which redox reactions occur based on the free energy gained from redox pairs. These redox gradients form both spatially and temporally as a result of differences in microbial processes, chemical composition of the environment, and oxidative potential. Common environments where redox gradients exist are coastal marshes, lakes, contaminant plumes, and soils.

Microbial oxidation of sulfur is the oxidation of sulfur by microorganisms to build their structural components. The oxidation of inorganic compounds is the strategy primarily used by chemolithotrophic microorganisms to obtain energy to survive, grow and reproduce. Some inorganic forms of reduced sulfur, mainly sulfide (H2S/HS−) and elemental sulfur (S0), can be oxidized by chemolithotrophic sulfur-oxidizing prokaryotes, usually coupled to the reduction of oxygen (O2) or nitrate (NO3−). Anaerobic sulfur oxidizers include photolithoautotrophs that obtain their energy from sunlight, hydrogen from sulfide, and carbon from carbon dioxide (CO2).

The hydrothermal vent microbial community includes all unicellular organisms that live and reproduce in a chemically distinct area around hydrothermal vents. These include organisms in the microbial mat, free floating cells, or bacteria in an endosymbiotic relationship with animals. Chemolithoautotrophic bacteria derive nutrients and energy from the geological activity at Hydrothermal vents to fix carbon into organic forms. Viruses are also a part of the hydrothermal vent microbial community and their influence on the microbial ecology in these ecosystems is a burgeoning field of research.













