Basalt is an aphanitic (fine-grained) extrusive igneous rock formed from the rapid cooling of low-viscosity lava rich in magnesium and iron exposed at or very near the surface of a rocky planet or moon. More than 90% of all volcanic rock on Earth is basalt. Rapid-cooling, fine-grained basalt is chemically equivalent to slow-cooling, coarse-grained gabbro. The eruption of basalt lava is observed by geologists at about 20 volcanoes per year. Basalt is also an important rock type on other planetary bodies in the Solar System. For example, the bulk of the plains of Venus, which cover ~80% of the surface, are basaltic; the lunar maria are plains of flood-basaltic lava flows; and basalt is a common rock on the surface of Mars.

The mineral olivine is a magnesium iron silicate with the chemical formula (Mg,Fe)2SiO4. It is a type of nesosilicate or orthosilicate. The primary component of the Earth's upper mantle, it is a common mineral in Earth's subsurface, but weathers quickly on the surface. For this reason, olivine has been proposed as a good candidate for accelerated weathering to sequester carbon dioxide from the Earth's oceans and atmosphere, as part of climate change mitigation. Olivine also has many other historical uses, such as the gemstone peridot, as well as industrial applications like metalworking processes.

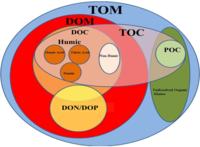
The carbon cycle is that part of the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycle. Carbon is the main component of biological compounds as well as a major component of many minerals such as limestone. The carbon cycle comprises a sequence of events that are key to making Earth capable of sustaining life. It describes the movement of carbon as it is recycled and reused throughout the biosphere, as well as long-term processes of carbon sequestration (storage) to and release from carbon sinks.

Kimberlite is an igneous rock and a rare variant of peridotite. It is most commonly known to be the main host matrix for diamonds. It is named after the town of Kimberley in South Africa, where the discovery of an 83.5-carat (16.70 g) diamond called the Star of South Africa in 1869 spawned a diamond rush and the digging of the open-pit mine called the Big Hole. Previously, the term kimberlite has been applied to olivine lamproites as Kimberlite II, however this has been in error.

Subduction is a geological process in which the oceanic lithosphere and some continental lithosphere is recycled into the Earth's mantle at convergent boundaries. Where the oceanic lithosphere of a tectonic plate converges with the less dense lithosphere of a second plate, the heavier plate dives beneath the second plate and sinks into the mantle. A region where this process occurs is known as a subduction zone, and its surface expression is known as an arc-trench complex. The process of subduction has created most of the Earth's continental crust. Rates of subduction are typically measured in centimeters per year, with rates of convergence as high as 11 cm/year.

A mantle plume is a proposed mechanism of convection within the Earth's mantle, hypothesized to explain anomalous volcanism. Because the plume head partially melts on reaching shallow depths, a plume is often invoked as the cause of volcanic hotspots, such as Hawaii or Iceland, and large igneous provinces such as the Deccan and Siberian Traps. Some such volcanic regions lie far from tectonic plate boundaries, while others represent unusually large-volume volcanism near plate boundaries.

Peridotite ( PERR-ih-doh-tyte, pə-RID-ə-) is a dense, coarse-grained igneous rock consisting mostly of the silicate minerals olivine and pyroxene. Peridotite is ultramafic, as the rock contains less than 45% silica. It is high in magnesium (Mg2+), reflecting the high proportions of magnesium-rich olivine, with appreciable iron. Peridotite is derived from Earth's mantle, either as solid blocks and fragments, or as crystals accumulated from magmas that formed in the mantle. The compositions of peridotites from these layered igneous complexes vary widely, reflecting the relative proportions of pyroxenes, chromite, plagioclase, and amphibole.

Oceanic crust is the uppermost layer of the oceanic portion of the tectonic plates. It is composed of the upper oceanic crust, with pillow lavas and a dike complex, and the lower oceanic crust, composed of troctolite, gabbro and ultramafic cumulates. The crust overlies the rigid uppermost layer of the mantle. The crust and the rigid upper mantle layer together constitute oceanic lithosphere.
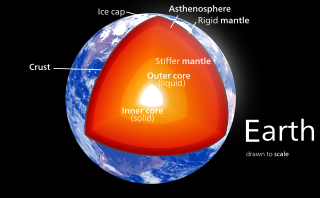
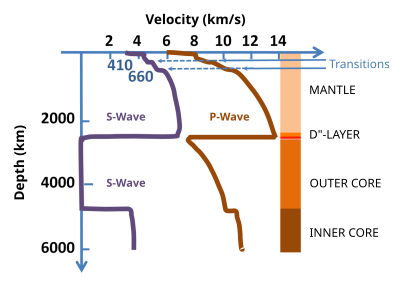
Earth's mantle is a layer of silicate rock between the crust and the outer core. It has a mass of 4.01×1024 kg (8.84×1024 lb) and thus makes up 67% of the mass of Earth. It has a thickness of 2,900 kilometers (1,800 mi) making up about 46% of Earth's radius and 84% of Earth's volume. It is predominantly solid but, on geologic time scales, it behaves as a viscous fluid, sometimes described as having the consistency of caramel. Partial melting of the mantle at mid-ocean ridges produces oceanic crust, and partial melting of the mantle at subduction zones produces continental crust.
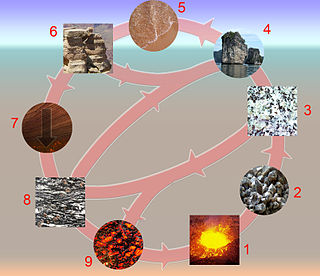
The rock cycle is a basic concept in geology that describes transitions through geologic time among the three main rock types: sedimentary, metamorphic, and igneous. Each rock type is altered when it is forced out of its equilibrium conditions. For example, an igneous rock such as basalt may break down and dissolve when exposed to the atmosphere, or melt as it is subducted under a continent. Due to the driving forces of the rock cycle, plate tectonics and the water cycle, rocks do not remain in equilibrium and change as they encounter new environments. The rock cycle explains how the three rock types are related to each other, and how processes change from one type to another over time. This cyclical aspect makes rock change a geologic cycle and, on planets containing life, a biogeochemical cycle.

Ocean island basalt (OIB) is a volcanic rock, usually basaltic in composition, erupted in oceans away from tectonic plate boundaries. Although ocean island basaltic magma is mainly erupted as basalt lava, the basaltic magma is sometimes modified by igneous differentiation to produce a range of other volcanic rock types, for example, rhyolite in Iceland, and phonolite and trachyte at the intraplate volcano Fernando de Noronha. Unlike mid-ocean ridge basalts (MORBs), which erupt at spreading centers (divergent plate boundaries), and volcanic arc lavas, which erupt at subduction zones (convergent plate boundaries), ocean island basalts are the result of intraplate volcanism. However, some ocean island basalt locations coincide with plate boundaries like Iceland, which sits on top of a mid-ocean ridge, and Samoa, which is located near a subduction zone.

Crustal recycling is a tectonic process by which surface material from the lithosphere is recycled into the mantle by subduction erosion or delamination. The subducting slabs carry volatile compounds and water into the mantle, as well as crustal material with an isotopic signature different from that of primitive mantle. Identification of this crustal signature in mantle-derived rocks is proof of crustal recycling.
The Deep Carbon Observatory (DCO) is a global research program designed to transform understanding of carbon's role in Earth. DCO is a community of scientists, including biologists, physicists, geoscientists and chemists, whose work crosses several traditional disciplinary lines to develop the new, integrative field of deep carbon science. To complement this research, the DCO's infrastructure includes public engagement and education, online and offline community support, innovative data management, and novel instrumentation development.

Magma oceans are vast fields of surface magma that exist during periods of a planet's or some natural satellite's accretion when the celestial body is completely or partly molten.
The Deep Earth Carbon Degassing (DECADE) project is an initiative to unite scientists around the world to make tangible advances towards quantifying the amount of carbon outgassed from the Earth's deep interior into the surface environment through naturally occurring processes. DECADE is an initiative within the Deep Carbon Observatory (DCO).

Marine biogeochemical cycles are biogeochemical cycles that occur within marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. These biogeochemical cycles are the pathways chemical substances and elements move through within the marine environment. In addition, substances and elements can be imported into or exported from the marine environment. These imports and exports can occur as exchanges with the atmosphere above, the ocean floor below, or as runoff from the land.
The geochemistry of carbon is the study of the transformations involving the element carbon within the systems of the Earth. To a large extent this study is organic geochemistry, but it also includes the very important carbon dioxide. Carbon is transformed by life, and moves between the major phases of the Earth, including the water bodies, atmosphere, and the rocky parts. Carbon is important in the formation of organic mineral deposits, such as coal, petroleum or natural gas. Most carbon is cycled through the atmosphere into living organisms and then respirated back into the atmosphere. However an important part of the carbon cycle involves the trapping of living matter into sediments. The carbon then becomes part of a sedimentary rock when lithification happens. Human technology or natural processes such as weathering, or underground life or water can return the carbon from sedimentary rocks to the atmosphere. From that point it can be transformed in the rock cycle into metamorphic rocks, or melted into igneous rocks. Carbon can return to the surface of the Earth by volcanoes or via uplift in tectonic processes. Carbon is returned to the atmosphere via volcanic gases. Carbon undergoes transformation in the mantle under pressure to diamond and other minerals, and also exists in the Earth's outer core in solution with iron, and may also be present in the inner core.

Mantle oxidation state (redox state) applies the concept of oxidation state in chemistry to the study of the Earth's mantle. The chemical concept of oxidation state mainly refers to the valence state of one element, while mantle oxidation state provides the degree of decreasing of increasing valence states of all polyvalent elements in mantle materials confined in a closed system. The mantle oxidation state is controlled by oxygen fugacity and can be benchmarked by specific groups of redox buffers.

The deep water cycle, or geologic water cycle, involves exchange of water with the mantle, with water carried down by subducting oceanic plates and returning through volcanic activity, distinct from the water cycle process that occurs above and on the surface of Earth. Some of the water makes it all the way to the lower mantle and may even reach the outer core. Mineral physics experiments show that hydrous minerals can carry water deep into the mantle in colder slabs and even "nominally anhydrous minerals" can store several oceans' worth of water.
Daniel James Frost, is a British Earth scientist, currently Professor of Experimental Geosciences at the University of Bayreuth. His research focuses on the nature of Earth's deep interior, including the chemistry of the mantle and how it led to the development of the atmosphere, and the physical and chemical processes through which planets form.