
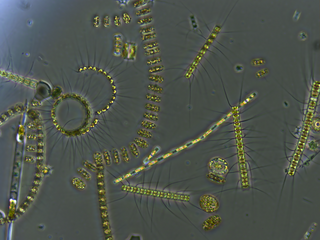
Coccolithophores, or coccolithophorids, are single-celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdom Protista, according to Robert Whittaker's five-kingdom system, or clade Hacrobia, according to a newer biological classification system. Within the Hacrobia, the coccolithophores are in the phylum or division Haptophyta, class Prymnesiophyceae. Coccolithophores are almost exclusively marine, are photosynthetic, and exist in large numbers throughout the sunlight zone of the ocean.
Phytoplankton are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater ecosystems. The name comes from the Greek words φυτόν, meaning 'plant', and πλαγκτός, meaning 'wanderer' or 'drifter'.

The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sediments. In other words, it is a biologically mediated process which results in the sequestering of carbon in the deep ocean away from the atmosphere and the land. The biological pump is the biological component of the "marine carbon pump" which contains both a physical and biological component. It is the part of the broader oceanic carbon cycle responsible for the cycling of organic matter formed mainly by phytoplankton during photosynthesis (soft-tissue pump), as well as the cycling of calcium carbonate (CaCO3) formed into shells by certain organisms such as plankton and mollusks (carbonate pump).

Coccoliths are individual plates or scales of calcium carbonate formed by coccolithophores and cover the cell surface arranged in the form of a spherical shell, called a coccosphere.

Gephyrocapsa huxleyi, formerly called Emiliania huxleyi, is a species of coccolithophore found in almost all ocean ecosystems from the equator to sub-polar regions, and from nutrient rich upwelling zones to nutrient poor oligotrophic waters. It is one of thousands of different photosynthetic plankton that freely drift in the photic zone of the ocean, forming the basis of virtually all marine food webs. It is studied for the extensive blooms it forms in nutrient-depleted waters after the reformation of the summer thermocline. Like other coccolithophores, E. huxleyi is a single-celled phytoplankton covered with uniquely ornamented calcite disks called coccoliths. Individual coccoliths are abundant in marine sediments although complete coccospheres are more unusual. In the case of E. huxleyi, not only the shell, but also the soft part of the organism may be recorded in sediments. It produces a group of chemical compounds that are very resistant to decomposition. These chemical compounds, known as alkenones, can be found in marine sediments long after other soft parts of the organisms have decomposed. Alkenones are most commonly used by earth scientists as a means to estimate past sea surface temperatures.
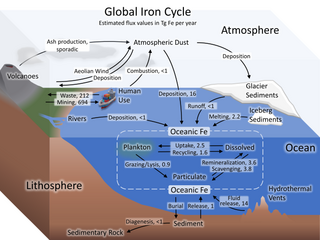
The iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient in primary productivity, and a limiting nutrient in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.
High-nutrient, low-chlorophyll (HNLC) regions are regions of the ocean where the abundance of phytoplankton is low and fairly constant despite the availability of macronutrients. Phytoplankton rely on a suite of nutrients for cellular function. Macronutrients are generally available in higher quantities in surface ocean waters, and are the typical components of common garden fertilizers. Micronutrients are generally available in lower quantities and include trace metals. Macronutrients are typically available in millimolar concentrations, while micronutrients are generally available in micro- to nanomolar concentrations. In general, nitrogen tends to be a limiting ocean nutrient, but in HNLC regions it is never significantly depleted. Instead, these regions tend to be limited by low concentrations of metabolizable iron. Iron is a critical phytoplankton micronutrient necessary for enzyme catalysis and electron transport.
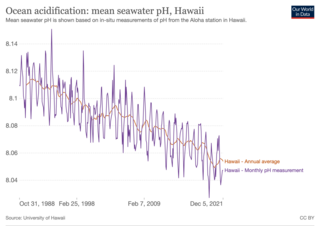
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Ocean acidification is a process that occurs when carbon dioxide (CO2) from the atmosphere is absorbed by seawater, leading to a decrease in pH levels. This results in an increase in acidity and a reduction in carbonate ions, which are crucial for marine organisms like corals, shellfish, and plankton to build their shells and skeletons. Over the past 200 years, the rapid increase in anthropogenic CO2 (carbon dioxide) production has led to an increase in the acidity of the Earth's oceans. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ocean acidification, with atmospheric carbon dioxide (CO2) levels exceeding 410 ppm (in 2020). CO2 from the atmosphere is absorbed by the oceans. This chemical reaction produces carbonic acid (H2CO3) which dissociates into a bicarbonate ion (HCO−3) and a hydrogen ion (H+). The presence of free hydrogen ions (H+) lowers the pH of the ocean, increasing acidity (this does not mean that seawater is acidic yet; it is still alkaline, with a pH higher than 8). Marine calcifying organisms, such as mollusks and corals, are especially vulnerable because they rely on calcium carbonate to build shells and skeletons.
Alkenones are long-chain unsaturated methyl and ethyl n-ketones produced by a few phytoplankton species of the class Prymnesiophyceae. Alkenones typically contain between 35 and 41 carbon atoms and with between two and four double bonds. Uniquely for biolipids, alkenones have a spacing of five methylene groups between double bonds, which are of the less common E configuration. The biological function of alkenones remains under debate although it is likely that they are storage lipids. Alkenones were first described in ocean sediments recovered from Walvis Ridge and then shortly afterwards in cultures of the marine coccolithophore Gephyrocapsa huxleyi. The earliest known occurrence of alkenones is during the Aptian 120 million years ago. They are used in organic geochemistry as a proxy for past sea surface temperature.

The CLAW hypothesis proposes a negative feedback loop that operates between ocean ecosystems and the Earth's climate. The hypothesis specifically proposes that particular phytoplankton that produce dimethyl sulfide are responsive to variations in climate forcing, and that these responses act to stabilise the temperature of the Earth's atmosphere. The CLAW hypothesis was originally proposed by Robert Jay Charlson, James Lovelock, Meinrat Andreae and Stephen G. Warren, and takes its acronym from the first letter of their surnames.

Siliceous ooze is a type of biogenic pelagic sediment located on the deep ocean floor. Siliceous oozes are the least common of the deep sea sediments, and make up approximately 15% of the ocean floor. Oozes are defined as sediments which contain at least 30% skeletal remains of pelagic microorganisms. Siliceous oozes are largely composed of the silica based skeletons of microscopic marine organisms such as diatoms and radiolarians. Other components of siliceous oozes near continental margins may include terrestrially derived silica particles and sponge spicules. Siliceous oozes are composed of skeletons made from opal silica SiO2·nH2O, as opposed to calcareous oozes, which are made from skeletons of calcium carbonate (CaCO3·nH2O) organisms (i.e. coccolithophores). Silica (Si) is a bioessential element and is efficiently recycled in the marine environment through the silica cycle. Distance from land masses, water depth and ocean fertility are all factors that affect the opal silica content in seawater and the presence of siliceous oozes.
Emiliania is a global coccolithophorid genus.

Particulate organic matter (POM) is a fraction of total organic matter operationally defined as that which does not pass through a filter pore size that typically ranges in size from 0.053 millimeters (53 μm) to 2 millimeters.

Marine biogenic calcification is the production of calcium carbonate by organisms in the global ocean.

Marine primary production is the chemical synthesis in the ocean of organic compounds from atmospheric or dissolved carbon dioxide. It principally occurs through the process of photosynthesis, which uses light as its source of energy, but it also occurs through chemosynthesis, which uses the oxidation or reduction of inorganic chemical compounds as its source of energy. Almost all life on Earth relies directly or indirectly on primary production. The organisms responsible for primary production are called primary producers or autotrophs.

Marine protists are defined by their habitat as protists that live in marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. Life originated as marine single-celled prokaryotes and later evolved into more complex eukaryotes. Eukaryotes are the more developed life forms known as plants, animals, fungi and protists. Protists are the eukaryotes that cannot be classified as plants, fungi or animals. They are mostly single-celled and microscopic. The term protist came into use historically as a term of convenience for eukaryotes that cannot be strictly classified as plants, animals or fungi. They are not a part of modern cladistics because they are paraphyletic.

Many protists have protective shells or tests, usually made from silica (glass) or calcium carbonate (chalk). Protists are a diverse group of eukaryote organisms that are not plants, animals, or fungi. They are typically microscopic unicellular organisms that live in water or moist environments.

Particulate inorganic carbon (PIC) can be contrasted with dissolved inorganic carbon (DIC), the other form of inorganic carbon found in the ocean. These distinctions are important in chemical oceanography. Particulate inorganic carbon is sometimes called suspended inorganic carbon. In operational terms, it is defined as the inorganic carbon in particulate form that is too large to pass through the filter used to separate dissolved inorganic carbon.

The Martin curve is a power law used by oceanographers to describe the export to the ocean floor of particulate organic carbon (POC). The curve is controlled with two parameters: the reference depth in the water column, and a remineralisation parameter which is a measure of the rate at which the vertical flux of POC attenuates. It is named after the American oceanographer John Martin.
Patricia Ana Matrai is a marine scientist known for her work on the cycling of sulfur. She is a senior research scientist at Bigelow Laboratory for Ocean Sciences.



















