Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive.

A diatom is any member of a large group comprising several genera of algae, specifically microalgae, found in the oceans, waterways and soils of the world. Living diatoms make up a significant portion of the Earth's biomass: they generate about 20 to 50 percent of the oxygen produced on the planet each year, take in over 6.7 billion tonnes of silicon each year from the waters in which they live, and constitute nearly half of the organic material found in the oceans. The shells of dead diatoms can reach as much as a half-mile deep on the ocean floor, and the entire Amazon basin is fertilized annually by 27 million tons of diatom shell dust transported by transatlantic winds from the African Sahara, much of it from the Bodélé Depression, which was once made up of a system of fresh-water lakes.

Chert is a hard, fine-grained sedimentary rock composed of microcrystalline or cryptocrystalline quartz, the mineral form of silicon dioxide (SiO2). Chert is characteristically of biological origin, but may also occur inorganically as a chemical precipitate or a diagenetic replacement, as in petrified wood.

Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
High-nutrient, low-chlorophyll (HNLC) regions are regions of the ocean where the abundance of phytoplankton is low and fairly constant despite the availability of macronutrients. Phytoplankton rely on a suite of nutrients for cellular function. Macronutrients are generally available in higher quantities in surface ocean waters, and are the typical components of common garden fertilizers. Micronutrients are generally available in lower quantities and include trace metals. Macronutrients are typically available in millimolar concentrations, while micronutrients are generally available in micro- to nanomolar concentrations. In general, nitrogen tends to be a limiting ocean nutrient, but in HNLC regions it is never significantly depleted. Instead, these regions tend to be limited by low concentrations of metabolizable iron. Iron is a critical phytoplankton micronutrient necessary for enzyme catalysis and electron transport.

Orthosilicic acid is an inorganic compound with the formula Si(OH)4. Although rarely observed, it is the key compound of silica and silicates and the precursor to other silicic acids [H2xSiOx+2]n. Silicic acids play important roles in biomineralization and technology.
The carbonate compensation depth (CCD) is the depth, in the oceans, at which the rate of supply of calcium carbonates matches the rate of solvation. That is, solvation 'compensates' supply. Below the CCD solvation is faster, so that carbonate particles dissolve and the carbonate shells (tests) of animals are not preserved. Carbonate particles cannot accumulate in the sediments where the sea floor is below this depth.

Pelagic sediment or pelagite is a fine-grained sediment that accumulates as the result of the settling of particles to the floor of the open ocean, far from land. These particles consist primarily of either the microscopic, calcareous or siliceous shells of phytoplankton or zooplankton; clay-size siliciclastic sediment; or some mixture of these. Trace amounts of meteoric dust and variable amounts of volcanic ash also occur within pelagic sediments. Based upon the composition of the ooze, there are three main types of pelagic sediments: siliceous oozes, calcareous oozes, and red clays.
Lithogenic silica (LSi) is silica (SiO2) derived from terrigenous rock (Igneous, metamorphic, and sedimentary), lithogenic sediments composed of the detritus of pre-existing rock, volcanic ejecta, extraterrestrial material, and minerals such silicate. Silica is the most abundant compound in the Earth's crust (59%) and the main component of almost every rock (>95%).

Radiolarite is a siliceous, comparatively hard, fine-grained, chert-like, and homogeneous sedimentary rock that is composed predominantly of the microscopic remains of radiolarians. This term is also used for indurated radiolarian oozes and sometimes as a synonym of radiolarian earth. However, radiolarian earth is typically regarded by Earth scientists to be the unconsolidated equivalent of a radiolarite. A radiolarian chert is well-bedded, microcrystalline radiolarite that has a well-developed siliceous cement or groundmass.

Marine sediment, or ocean sediment, or seafloor sediment, are deposits of insoluble particles that have accumulated on the seafloor. These particles have their origins in soil and rocks and have been transported from the land to the sea, mainly by rivers but also by dust carried by wind and by the flow of glaciers into the sea. Additional deposits come from marine organisms and chemical precipitation in seawater, as well as from underwater volcanoes and meteorite debris.

Siliceous ooze is a type of biogenic pelagic sediment located on the deep ocean floor. Siliceous oozes are the least common of the deep sea sediments, and make up approximately 15% of the ocean floor. Oozes are defined as sediments which contain at least 30% skeletal remains of pelagic microorganisms. Siliceous oozes are largely composed of the silica based skeletons of microscopic marine organisms such as diatoms and radiolarians. Other components of siliceous oozes near continental margins may include terrestrially derived silica particles and sponge spicules. Siliceous oozes are composed of skeletons made from opal silica SiO2·nH2O, as opposed to calcareous oozes, which are made from skeletons of calcium carbonate (CaCO3·nH2O) organisms (i.e. coccolithophores). Silica (Si) is a bioessential element and is efficiently recycled in the marine environment through the silica cycle. Distance from land masses, water depth and ocean fertility are all factors that affect the opal silica content in seawater and the presence of siliceous oozes.
The Southern Pacific Gyre is part of the Earth's system of rotating ocean currents, bounded by the Equator to the north, Australia to the west, the Antarctic Circumpolar Current to the south, and South America to the east. The center of the South Pacific Gyre is the oceanic pole of inaccessibility, the site on Earth farthest from any continents and productive ocean regions and is regarded as Earth's largest oceanic desert. With an area of 37 million square kilometres it makes up ~10 % of the Earth's ocean surface. The gyre, as with Earth's other four gyres, contains an area with elevated concentrations of pelagic plastics, chemical sludge, and other debris known as the South Pacific garbage patch.
Reverse weathering generally refers to the formation of a clay neoformation that utilizes cations and alkalinity in a process unrelated to the weathering of silicates. More specifically reverse weathering refers to the formation of authigenic clay minerals from the reaction of 1) biogenic silica with aqueous cations or cation bearing oxides or 2) cation poor precursor clays with dissolved cations or cation bearing oxides.
Nutrient cycling in the Columbia River Basin involves the transport of nutrients through the system, as well as transformations from among dissolved, solid, and gaseous phases, depending on the element. The elements that constitute important nutrient cycles include macronutrients such as nitrogen, silicate, phosphorus, and micronutrients, which are found in trace amounts, such as iron. Their cycling within a system is controlled by many biological, chemical, and physical processes.
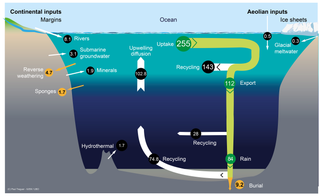
The silica cycle is the biogeochemical cycle in which biogenic silica is transported between the Earth's systems. Silicon is considered a bioessential element and is one of the most abundant elements on Earth. The silica cycle has significant overlap with the carbon cycle and plays an important role in the sequestration of carbon through continental weathering, biogenic export and burial as oozes on geologic timescales.

Many protists have protective shells or tests, usually made from silica (glass) or calcium carbonate (chalk). Protists are a diverse group of eukaryote organisms that are not plants, animals, or fungi. They are typically microscopic unicellular organisms that live in water or moist environments.

In geology, silicification is a petrification process in which silica-rich fluids seep into the voids of Earth materials, e.g., rocks, wood, bones, shells, and replace the original materials with silica (SiO2). Silica is a naturally existing and abundant compound found in organic and inorganic materials, including Earth's crust and mantle. There are a variety of silicification mechanisms. In silicification of wood, silica permeates into and occupies cracks and voids in wood such as vessels and cell walls. The original organic matter is retained throughout the process and will gradually decay through time. In the silicification of carbonates, silica replaces carbonates by the same volume. Replacement is accomplished through the dissolution of original rock minerals and the precipitation of silica. This leads to a removal of original materials out of the system. Depending on the structures and composition of the original rock, silica might replace only specific mineral components of the rock. Silicic acid (H4SiO4) in the silica-enriched fluids forms lenticular, nodular, fibrous, or aggregated quartz, opal, or chalcedony that grows within the rock. Silicification happens when rocks or organic materials are in contact with silica-rich surface water, buried under sediments and susceptible to groundwater flow, or buried under volcanic ashes. Silicification is often associated with hydrothermal processes. Temperature for silicification ranges in various conditions: in burial or surface water conditions, temperature for silicification can be around 25°−50°; whereas temperatures for siliceous fluid inclusions can be up to 150°−190°. Silicification could occur during a syn-depositional or a post-depositional stage, commonly along layers marking changes in sedimentation such as unconformities or bedding planes.
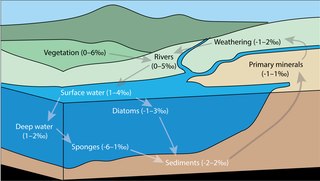
Silicon isotope biogeochemistry is the study of environmental processes using the relative abundance of Si isotopes. As the relative abundance of Si stable isotopes varies among different natural materials, the differences in abundance can be used to trace the source of Si, and to study biological, geological, and chemical processes. The study of stable isotope biogeochemistry of Si aims to quantify the different Si fluxes in the global biogeochemical silicon cycle, to understand the role of biogenic silica within the global Si cycle, and to investigate the applications and limitations of the sedimentary Si record as an environmental and palaeoceanographic proxy.
Biogenous ooze is marine sediment that accumulates on the seafloor and is a byproduct of the death and sink of the skeletal remains of marine organisms.