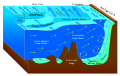
Benthos, also known as benthon, is the community of organisms that live on, in, or near the bottom of a sea, river, lake, or stream, also known as the benthic zone. This community lives in or near marine or freshwater sedimentary environments, from tidal pools along the foreshore, out to the continental shelf, and then down to the abyssal depths.
The pelagic zone consists of the water column of the open ocean and can be further divided into regions by depth. The word pelagic is derived from Ancient Greek πέλαγος (pélagos) 'open sea'. The pelagic zone can be thought of as an imaginary cylinder or water column between the surface of the sea and the bottom. Conditions in the water column change with depth: pressure increases; temperature and light decrease; salinity, oxygen, micronutrients all change. In a manner analogous to stratification in the Earth's atmosphere, the water column can be divided vertically into up to five different layers, with the number of layers depending on the depth of the water.

The littoral zone, also called litoral or nearshore, is the part of a sea, lake, or river that is close to the shore. In coastal ecology, the littoral zone includes the intertidal zone extending from the high water mark, to coastal areas that are permanently submerged — known as the foreshore — and the terms are often used interchangeably. However, the geographical meaning of littoral zone extends well beyond the intertidal zone to include all neritic waters within the bounds of continental shelves.

Bottom trawling is trawling along the seafloor. It is also referred to as "dragging". The scientific community divides bottom trawling into benthic trawling and demersal trawling. Benthic trawling is towing a net at the very bottom of the ocean and demersal trawling is towing a net just above the benthic zone. Bottom trawling can be contrasted with midwater trawling, where a net is towed higher in the water column. Midwater trawling catches pelagic fish such as anchovies and mackerel, whereas bottom trawling targets both bottom-living fish (groundfish) and semi-pelagic species such as cod, squid, shrimp, and rockfish.

The seabed is the bottom of the ocean. All floors of the ocean are known as 'seabeds'.
The abyssal zone or abyssopelagic zone is a layer of the pelagic zone of the ocean. The word abyss comes from the Greek word ἄβυσσος (ábussos), meaning "bottomless". At depths of 4,000–6,000 m (13,000–20,000 ft), this zone remains in perpetual darkness. It covers 83% of the total area of the ocean and 60% of Earth's surface. The abyssal zone has temperatures around 2–3 °C (36–37 °F) through the large majority of its mass. The water pressure can reach up to 76 MPa.

Pulley Ridge is a mesophotic coral reef system off the shores of the continental United States. The reef rests on sunken barrier islands and lies 100 miles west of the Tortugas Ecological Reserve and stretches north about 60 miles at depths ranging from 60 to 80 meters. Pulley Ridge was originally discovered in 1950 during a dredging operation conducted by an academic group from Texas. While well known to fishermen, this remarkable habitat remained undiscovered by scientists until 1999 when the U.S. Geological Survey (USGS) and graduate students from the University of South Florida happened upon it. This reef system, like other mesophotic ecosystems, is inhabited by photosynthesizing corals and algae that are adapted to low-light environments. It is habitat for numerous species of bottom fish including Epinephelus morio spawning area.

Pelagic fish live in the pelagic zone of ocean or lake waters—being neither close to the bottom nor near the shore—in contrast with demersal fish that live on or near the bottom, and reef fish that are associated with coral reefs.

Demersal fish, also known as groundfish, live and feed on or near the bottom of seas or lakes. They occupy the sea floors and lake beds, which usually consist of mud, sand, gravel or rocks. In coastal waters, they are found on or near the continental shelf, and in deep waters, they are found on or near the continental slope or along the continental rise. They are not generally found in the deepest waters, such as abyssal depths or on the abyssal plain, but they can be found around seamounts and islands. The word demersal comes from the Latin demergere, which means to sink.
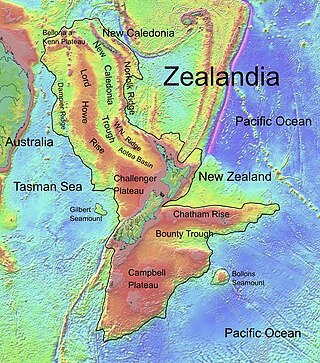
The Lord Howe Rise is a deep sea plateau which extends from south west of New Caledonia to the Challenger Plateau, west of New Zealand in the south west of the Pacific Ocean. To its west is the Tasman Basin and to the east is the New Caledonia Basin. Lord Howe Rise has a total area of about 1,500,000 km2 (580,000 sq mi), and generally lies about 750 to 1,200 metres under water. It is part of Zealandia, a much larger continent that is now mostly submerged, and so is composed of continental crust. Some have included the 3,500 m (11,500 ft) deep New Caledonia Basin as within the rise, given its continental crust origin, and this would give a larger total area of 1,950,000 km2 (750,000 sq mi).

Marine ecosystems are the largest of Earth's aquatic ecosystems and exist in waters that have a high salt content. These systems contrast with freshwater ecosystems, which have a lower salt content. Marine waters cover more than 70% of the surface of the Earth and account for more than 97% of Earth's water supply and 90% of habitable space on Earth. Seawater has an average salinity of 35 parts per thousand of water. Actual salinity varies among different marine ecosystems. Marine ecosystems can be divided into many zones depending upon water depth and shoreline features. The oceanic zone is the vast open part of the ocean where animals such as whales, sharks, and tuna live. The benthic zone consists of substrates below water where many invertebrates live. The intertidal zone is the area between high and low tides. Other near-shore (neritic) zones can include mudflats, seagrass meadows, mangroves, rocky intertidal systems, salt marshes, coral reefs, lagoons. In the deep water, hydrothermal vents may occur where chemosynthetic sulfur bacteria form the base of the food web.

Marine sediment, or ocean sediment, or seafloor sediment, are deposits of insoluble particles that have accumulated on the seafloor. These particles either have their origins in soil and rocks and have been transported from the land to the sea, mainly by rivers but also by dust carried by wind and by the flow of glaciers into the sea, or they are biogenic deposits from marine organisms or from chemical precipitation in seawater, as well as from underwater volcanoes and meteorite debris.

The Integrated Marine and Coastal Regionalisation of Australia (IMCRA), formerly the Interim Marine and Coastal Regionalisation for Australia, is a biogeographic regionalisation of the oceanic waters of Australia's exclusive economic zone (EEZ). As of 2008, the most recent version is IMCRA Version 4.0.
This is a glossary of terms used in fisheries, fisheries management and fisheries science.

A deep-sea community is any community of organisms associated by a shared habitat in the deep sea. Deep sea communities remain largely unexplored, due to the technological and logistical challenges and expense involved in visiting this remote biome. Because of the unique challenges, it was long believed that little life existed in this hostile environment. Since the 19th century however, research has demonstrated that significant biodiversity exists in the deep sea.
The benthic boundary layer (BBL) is the layer of water directly above the sediment at the bottom of a body of water. Through specific sedimentation processes, certain organisms are able to live in this deep layer of water. The BBL is generated by the friction of the water moving over the surface of the substrate, which decrease the water current significantly in this layer. The thickness of this zone is determined by many factors, including the Coriolis force. The benthic organisms and processes in this boundary layer echo the water column above them.

A marine habitat is a habitat that supports marine life. Marine life depends in some way on the saltwater that is in the sea. A habitat is an ecological or environmental area inhabited by one or more living species. The marine environment supports many kinds of these habitats.

Jelly-falls are marine carbon cycling events whereby gelatinous zooplankton, primarily cnidarians, sink to the seafloor and enhance carbon and nitrogen fluxes via rapidly sinking particulate organic matter. These events provide nutrition to benthic megafauna and bacteria. Jelly-falls have been implicated as a major “gelatinous pathway” for the sequestration of labile biogenic carbon through the biological pump. These events are common in protected areas with high levels of primary production and water quality suitable to support cnidarian species. These areas include estuaries and several studies have been conducted in fjords of Norway.
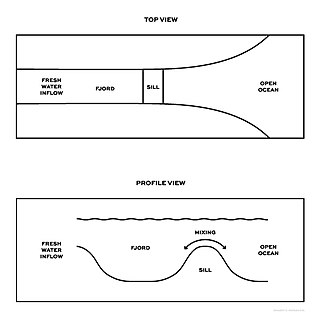
An aquatic sill is a sea floor barrier of relatively shallow depth that restricts water movement between benthic zones of an oceanic basin or lake bottom. There are roughly 400 sills in the Earth's oceans, covering 0.01% of the seafloor. A classic example is the Strait of Gibraltar Gateway between the Mediterranean sea and the Atlantic Ocean.

Benthic-pelagic coupling are processes that connect the benthic zone and the pelagic zone through the exchange of energy, mass, or nutrients. These processes play a prominent role in both freshwater and marine ecosystems and are influenced by a number of chemical, biological, and physical forces that are crucial to functions from nutrient cycling to energy transfer in food webs.