The carbon cycle is that part of the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycle. Carbon is the main component of biological compounds as well as a major component of many rocks such as limestone. The carbon cycle comprises a sequence of events that are key to making Earth capable of sustaining life. It describes the movement of carbon as it is recycled and reused throughout the biosphere, as well as long-term processes of carbon sequestration (storage) to and release from carbon sinks.

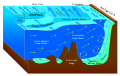
The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sediments. In other words, it is a biologically mediated process which results in the sequestering of carbon in the deep ocean away from the atmosphere and the land. The biological pump is the biological component of the "marine carbon pump" which contains both a physical and biological component. It is the part of the broader oceanic carbon cycle responsible for the cycling of organic matter formed mainly by phytoplankton during photosynthesis (soft-tissue pump), as well as the cycling of calcium carbonate (CaCO3) formed into shells by certain organisms such as plankton and mollusks (carbonate pump).
A hydrogen ion is created when a hydrogen atom loses an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle-free space. Due to its extremely high charge density of approximately 2×1010 times that of a sodium ion, the bare hydrogen ion cannot exist freely in solution as it readily hydrates, i.e., bonds quickly. The hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes. Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions.

In oceanic biogeochemistry, the solubility pump is a physico-chemical process that transports carbon as dissolved inorganic carbon (DIC) from the ocean's surface to its interior.

In oceanic biogeochemistry, the continental shelf pump is proposed to operate in the shallow waters of the continental shelves, acting as a mechanism to transport carbon from surface waters to the interior of the adjacent deep ocean.

Dissolved inorganic carbon (DIC) is the sum of the aqueous species of inorganic carbon in a solution. Carbon compounds can be distinguished as either organic or inorganic, and as dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as – proteins, lipids, carbohydrates, and nucleic acids.
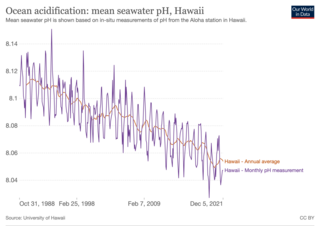
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ocean acidification, with atmospheric carbon dioxide levels exceeding 422 ppm. CO2 from the atmosphere is absorbed by the oceans. This chemical reaction produces carbonic acid which dissociates into a bicarbonate ion and a hydrogen ion. The presence of free hydrogen ions lowers the pH of the ocean, increasing acidity. Marine calcifying organisms, such as mollusks and corals, are especially vulnerable because they rely on calcium carbonate to build shells and skeletons.
The carbonate compensation depth (CCD) is the depth, in the oceans, at which the rate of supply of calcium carbonates matches the rate of solvation. That is, solvation 'compensates' supply. Below the CCD solvation is faster, so that carbonate particles dissolve and the carbonate shells (tests) of animals are not preserved. Carbonate particles cannot accumulate in the sediments where the sea floor is below this depth.
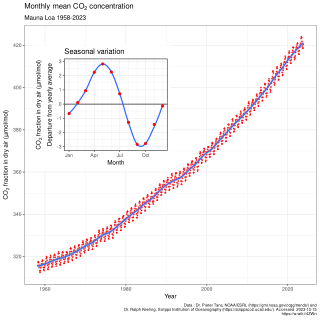
In Earth's atmosphere, carbon dioxide is a trace gas that plays an integral part in the greenhouse effect, carbon cycle, photosynthesis and oceanic carbon cycle. It is one of three main greenhouse gases in the atmosphere of Earth. The concentration of carbon dioxide in the atmosphere reach 427 ppm (0.04%) in 2024. This is an increase of 50% since the start of the Industrial Revolution, up from 280 ppm during the 10,000 years prior to the mid-18th century. The increase is due to human activity.

The airborne fraction is a scaling factor defined as the ratio of the annual increase in atmospheric CO
2 to the CO
2 emissions from human sources. It represents the proportion of human emitted CO2 that remains in the atmosphere. Observations over the past six decades show that the airborne fraction has remained relatively stable at around 45%. This indicates that the land and ocean's capacity to absorb CO2 has kept up with the rise in human CO2 emissions, despite the occurrence of notable interannual and sub-decadal variability, which is predominantly driven by the land's ability to absorb CO2. There is some evidence for a recent increase in airborne fraction, which would imply a faster increase in atmospheric CO
2 for a given rate of human fossil-fuel burning. Changes in carbon sinks can affect the airborne fraction as well.

The carbonate–silicate geochemical cycle, also known as the inorganic carbon cycle, describes the long-term transformation of silicate rocks to carbonate rocks by weathering and sedimentation, and the transformation of carbonate rocks back into silicate rocks by metamorphism and volcanism. Carbon dioxide is removed from the atmosphere during burial of weathered minerals and returned to the atmosphere through volcanism. On million-year time scales, the carbonate-silicate cycle is a key factor in controlling Earth's climate because it regulates carbon dioxide levels and therefore global temperature.
Marine chemistry, also known as ocean chemistry or chemical oceanography, is the study of the chemical composition and processes of the world’s oceans, including the interactions between seawater, the atmosphere, the seafloor, and marine organisms. This field encompasses a wide range of topics, such as the cycling of elements like carbon, nitrogen, and phosphorus, the behavior of trace metals, and the study of gases and nutrients in marine environments. Marine chemistry plays a crucial role in understanding global biogeochemical cycles, ocean circulation, and the effects of human activities, such as pollution and climate change, on oceanic systems. It is influenced by plate tectonics and seafloor spreading, turbidity, currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology.
The Revelle factor (buffer factor) is the ratio of instantaneous change in carbon dioxide (CO2) to the change in total dissolved inorganic carbon (DIC), and is a measure of the resistance to atmospheric CO2 being absorbed by the ocean surface layer. The buffer factor is used to examine the distribution of CO2 between the atmosphere and the ocean, and measures the amount of CO2 that can be dissolved in the mixed surface layer. It is named after the oceanographer Roger Revelle. The Revelle factor describes the ocean's ability to uptake atmospheric CO2, and is typically referenced in global carbon budget analysis and anthropogenic climate change studies.

The atmospheric carbon cycle accounts for the exchange of gaseous carbon compounds, primarily carbon dioxide, between Earth's atmosphere, the oceans, and the terrestrial biosphere. It is one of the faster components of the planet's overall carbon cycle, supporting the exchange of more than 200 billion tons of carbon in and out of the atmosphere throughout the course of each year. Atmospheric concentrations of CO2 remain stable over longer timescales only when there exists a balance between these two flows. Methane, Carbon monoxide (CO), and other human-made compounds are present in smaller concentrations and are also part of the atmospheric carbon cycle.

The oceanic carbon cycle is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic carbon and organic carbon. Part of the marine carbon cycle transforms carbon between non-living and living matter.
The Southern Ocean Carbon and Climate Observations and Modeling (SOCCOM) project is a large scale National Science Foundation funded research project based at Princeton University that started in September 2014. The project aims to increase the understanding of the Southern Ocean and the role it plays in factors such as climate, as well as educate new scientists with oceanic observation.

Marine biogenic calcification is the production of calcium carbonate by organisms in the global ocean.

The Arctic Ocean covers an area of 14,056,000 square kilometers, and supports a diverse and important socioeconomic food web of organisms, despite its average water temperature being 32 degrees Fahrenheit. Over the last three decades, the Arctic Ocean has experienced drastic changes due to climate change. One of the changes is in the acidity levels of the ocean, which have been consistently increasing at twice the rate of the Pacific and Atlantic oceans. Arctic Ocean acidification is a result of feedback from climate system mechanisms, and is having negative impacts on Arctic Ocean ecosystems and the organisms that live within them.

Particulate inorganic carbon (PIC) can be contrasted with dissolved inorganic carbon (DIC), the other form of inorganic carbon found in the ocean. These distinctions are important in chemical oceanography. Particulate inorganic carbon is sometimes called suspended inorganic carbon. In operational terms, it is defined as the inorganic carbon in particulate form that is too large to pass through the filter used to separate dissolved inorganic carbon.
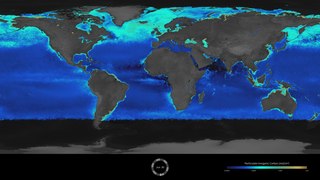
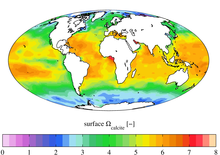
The Great Calcite Belt (GCB) refers to a region of the ocean where there are high concentrations of calcite, a mineral form of calcium carbonate. The belt extends over a large area of the Southern Ocean surrounding Antarctica. The calcite in the Great Calcite Belt is formed by tiny marine organisms called coccolithophores, which build their shells out of calcium carbonate. When these organisms die, their shells sink to the bottom of the ocean, and over time, they accumulate to form a thick layer of calcite sediment.