
Hypoxanthine is a naturally occurring purine derivative. It is occasionally found as a constituent of nucleic acids, where it is present in the anticodon of tRNA in the form of its nucleoside inosine. It has a tautomer known as 6-hydroxypurine. Hypoxanthine is a necessary additive in certain cell, bacteria, and parasite cultures as a substrate and nitrogen source. For example, it is commonly a required reagent in malaria parasite cultures, since Plasmodium falciparum requires a source of hypoxanthine for nucleic acid synthesis and energy metabolism.
Antimalarial medications or simply antimalarials are a type of antiparasitic chemical agent, often naturally derived, that can be used to treat or to prevent malaria, in the latter case, most often aiming at two susceptible target groups, young children and pregnant women. As of 2018, modern treatments, including for severe malaria, continued to depend on therapies deriving historically from quinine and artesunate, both parenteral (injectable) drugs, expanding from there into the many classes of available modern drugs. Incidence and distribution of the disease is expected to remain high, globally, for many years to come; moreover, known antimalarial drugs have repeatedly been observed to elicit resistance in the malaria parasite—including for combination therapies featuring artemisinin, a drug of last resort, where resistance has now been observed in Southeast Asia. As such, the needs for new antimalarial agents and new strategies of treatment remain important priorities in tropical medicine. As well, despite very positive outcomes from many modern treatments, serious side effects can impact some individuals taking standard doses.

Plasmodium is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of Plasmodium species involve development in a blood-feeding insect host which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue before entering the bloodstream to infect red blood cells. The ensuing destruction of host red blood cells can result in disease, called malaria. During this infection, some parasites are picked up by a blood-feeding insect, continuing the life cycle.
Artemether is a medication used for the treatment of malaria. The injectable form is specifically used for severe malaria rather than quinine. In adults, it may not be as effective as artesunate. It is given by injection in a muscle. It is also available by mouth in combination with lumefantrine, known as artemether/lumefantrine.
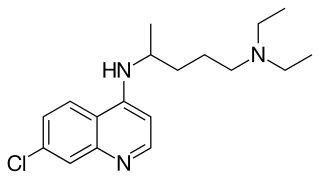
Chloroquine is a medication primarily used to prevent and treat malaria in areas where malaria remains sensitive to its effects. Certain types of malaria, resistant strains, and complicated cases typically require different or additional medication. Chloroquine is also occasionally used for amebiasis that is occurring outside the intestines, rheumatoid arthritis, and lupus erythematosus. While it has not been formally studied in pregnancy, it appears safe. It was studied to treat COVID-19 early in the pandemic, but these studies were largely halted in the summer of 2020, and is not recommended for this purpose. It is taken by mouth.

Plasmodium vivax is a protozoal parasite and a human pathogen. This parasite is the most frequent and widely distributed cause of recurring malaria. Although it is less virulent than Plasmodium falciparum, the deadliest of the five human malaria parasites, P. vivax malaria infections can lead to severe disease and death, often due to splenomegaly. P. vivax is carried by the female Anopheles mosquito; the males do not bite.

Fosmidomycin is an antibiotic that was originally isolated from culture broths of bacteria of the genus Streptomyces. It specifically inhibits DXP reductoisomerase, a key enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. It is a structural analogue of 2-C-methyl-D-erythrose 4-phosphate. It inhibits the E. coli enzyme with a KI value of 38 nM (4), MTB at 80 nM, and the Francisella enzyme at 99 nM. Several mutations in the E. coli DXP reductoisomerase were found to confer resistance to fosmidomycin.
An apicoplast is a derived non-photosynthetic plastid found in most Apicomplexa, including Toxoplasma gondii, Plasmodium falciparum and other Plasmodium spp., but not in others such as Cryptosporidium. It originated from algae through secondary endosymbiosis. The apicoplast is surrounded by four membranes within the outermost part of the endomembrane system. The apicoplast hosts important metabolic pathways like fatty acid synthesis, isoprenoid precursor synthesis and parts of the heme biosynthetic pathway
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP.
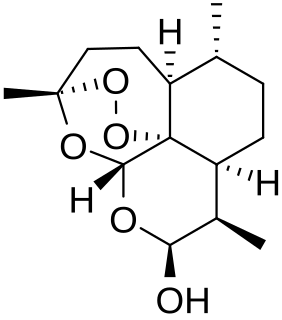
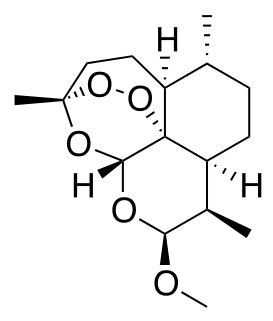
Dihydroartemisinin is a drug used to treat malaria. Dihydroartemisinin is the active metabolite of all artemisinin compounds and is also available as a drug in itself. It is a semi-synthetic derivative of artemisinin and is widely used as an intermediate in the preparation of other artemisinin-derived antimalarial drugs. It is sold commercially in combination with piperaquine and has been shown to be equivalent to artemether/lumefantrine.
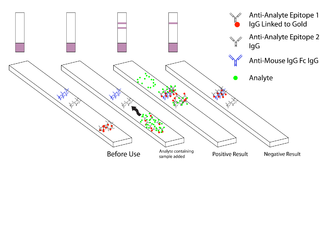
Malaria antigen detection tests are a group of commercially available rapid diagnostic tests of the rapid antigen test type that allow quick diagnosis of malaria by people who are not otherwise skilled in traditional laboratory techniques for diagnosing malaria or in situations where such equipment is not available. There are currently over 20 such tests commercially available. The first malaria antigen suitable as target for such a test was a soluble glycolytic enzyme Glutamate dehydrogenase. None of the rapid tests are currently as sensitive as a thick blood film, nor as cheap. A major drawback in the use of all current dipstick methods is that the result is essentially qualitative. In many endemic areas of tropical Africa, however, the quantitative assessment of parasitaemia is important, as a large percentage of the population will test positive in any qualitative assay.

6-Phosphogluconolactonase is a cytosolic enzyme found in all organisms that catalyzes the hydrolysis of 6-phosphogluconolactone to 6-phosphogluconic acid in the oxidative phase of the pentose phosphate pathway. The tertiary structure of 6PGL employs an α/β hydrolase fold, with active site residues clustered on the loops of the α-helices. Based on the crystal structure of the enzyme, the mechanism is proposed to be dependent on proton transfer by a histidine residue in the active site. 6PGL selectively catalyzes the hydrolysis of δ-6-phosphogluconolactone, and has no activity on the γ isomer.

The history of malaria stretches from its prehistoric origin as a zoonotic disease in the primates of Africa through to the 21st century. A widespread and potentially lethal human infectious disease, at its peak malaria infested every continent, except Antarctica. Its prevention and treatment have been targeted in science and medicine for hundreds of years. Since the discovery of the Plasmodium parasites which cause it, research attention has focused on their biology, as well as that of the mosquitoes which transmit the parasites.

Haemozoin is a disposal product formed from the digestion of blood by some blood-feeding parasites. These hematophagous organisms such as malaria parasites, Rhodnius and Schistosoma digest haemoglobin and release high quantities of free heme, which is the non-protein component of haemoglobin. Heme is a prosthetic group consisting of an iron atom contained in the center of a heterocyclic porphyrin ring. Free heme is toxic to cells, so the parasites convert it into an insoluble crystalline form called hemozoin. In malaria parasites, hemozoin is often called malaria pigment.
Chromera velia, also known as a "chromerid", is a unicellular photosynthetic organism in the superphylum Alveolata. It is of interest in the study of apicomplexan parasites, specifically their evolution and accordingly, their unique vulnerabilities to drugs.
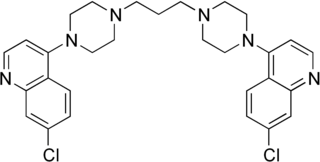
Piperaquine is an antiparasitic drug used in combination with dihydroartemisinin to treat malaria. Piperaquine was developed under the Chinese National Malaria Elimination Programme in the 1960s and was adopted throughout China as a replacement for the structurally similar antimalarial drug chloroquine. Due to widespread parasite resistance to piperaquine, the drug fell out of use as a monotherapy, and is instead used as a partner drug for artemisinin combination therapy. Piperaquine kills parasites by disrupting the detoxification of host heme.
DNA-binding proteins from starved cells (DPS) are bacterial proteins that belong to the ferritin superfamily and are characterized by strong similarities but also distinctive differences with respect to "canonical" ferritins.
Plasmodium cynomolgi is an apicomplexan parasite that infects mosquitoes and Asian Old World monkeys. This species has been used as a model for human Plasmodium vivax because Plasmodium cynomolgi shares the same life cycle and some important biological features with P. vivax.
Asif Mohmmed is an Indian cell biologist, parasitologist and a professor at the International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi. At ICGEB, he leads a research group on Parasite Cell Biology and is one of the key collaborators of the Tewari Lab at the School of Life Science of the University of Nottingham. He is known for his studies on Plasmodium falciparum proteases with regard to cellular Stress and parasite cell-death and protein-trafficking machinery in the pathogen, as well as the development of new anti-malarial drugs. His studies have been documented by way of a number of articles and ResearchGate, an online repository of scientific articles has listed 73 of them. The Department of Biotechnology of the Government of India awarded him the National Bioscience Award for Career Development, one of the highest Indian science awards, for his contributions to biosciences, in 2011.
Leslie Leiserowitz is an Israeli chemist and crystallographer.














