
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης 'violet-coloured'.
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.
The pedosphere is the outermost layer of the Earth that is composed of soil and subject to soil formation processes. It exists at the interface of the lithosphere, atmosphere, hydrosphere and biosphere. The pedosphere is the skin of the Earth and only develops when there is a dynamic interaction between the atmosphere, biosphere, lithosphere and the hydrosphere. The pedosphere is the foundation of terrestrial life on Earth.

Iodic acid is a white water-soluble solid with the chemical formula HIO3. Its robustness contrasts with the instability of chloric acid and bromic acid. Iodic acid features iodine in the oxidation state +5 and is one of the most stable oxo-acids of the halogens. When heated, samples dehydrate to give iodine pentoxide. On further heating, the iodine pentoxide further decomposes, giving a mix of iodine, oxygen and lower oxides of iodine.

The important sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration. The global sulfur cycle involves the transformations of sulfur species through different oxidation states, which play an important role in both geological and biological processes. Steps of the sulfur cycle are:

The Briggs–Rauscher oscillating reaction is one of a small number of known oscillating chemical reactions. It is especially well suited for demonstration purposes because of its visually striking colour changes: the freshly prepared colourless solution slowly turns an amber colour, then suddenly changes to a very dark blue. This slowly fades to colourless and the process repeats, about ten times in the most popular formulation, before ending as a dark blue liquid smelling strongly of iodine.

The microbial loop describes a trophic pathway where, in aquatic systems, dissolved organic carbon (DOC) is returned to higher trophic levels via its incorporation into bacterial biomass, and then coupled with the classic food chain formed by phytoplankton-zooplankton-nekton. In soil systems, the microbial loop refers to soil carbon. The term microbial loop was coined by Farooq Azam, Tom Fenchel et al. in 1983 to include the role played by bacteria in the carbon and nutrient cycles of the marine environment.

The phosphorus cycle is the biogeochemical cycle that describes the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the movement of phosphorus, because phosphorus and phosphorus-based compounds are usually solids at the typical ranges of temperature and pressure found on Earth. The production of phosphine gas occurs in only specialized, local conditions. Therefore, the phosphorus cycle should be viewed from whole Earth system and then specifically focused on the cycle in terrestrial and aquatic systems.
Organoiodine chemistry is the study of the synthesis and properties of organoiodine compounds, or organoiodides, organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt.
Iodine is an essential trace element in biological systems. It has the distinction of being the heaviest element commonly needed by living organisms as well as the second-heaviest known to be used by any form of life. It is a component of biochemical pathways in organisms from all biological kingdoms, suggesting its fundamental significance throughout the evolutionary history of life.

Marine biogeochemical cycles are biogeochemical cycles that occur within marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. These biogeochemical cycles are the pathways chemical substances and elements move through within the marine environment. In addition, substances and elements can be imported into or exported from the marine environment. These imports and exports can occur as exchanges with the atmosphere above, the ocean floor below, or as runoff from the land.

The boron cycle is the biogeochemical cycle of boron through the atmosphere, lithosphere, biosphere, and hydrosphere.

The arsenic (As) cycle is the biogeochemical cycle of natural and anthropogenic exchanges of arsenic terms through the atmosphere, lithosphere, pedosphere, hydrosphere, and biosphere. Although arsenic is naturally abundant in the Earth's crust, long-term exposure and high concentrations of arsenic can be detrimental to human health.
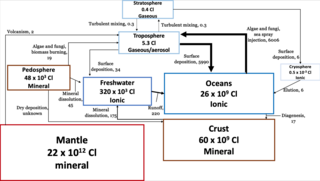
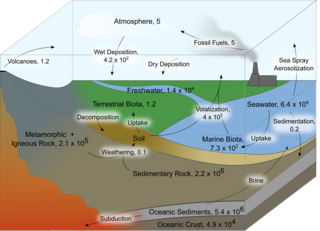
The chlorine cycle (Cl) is the biogeochemical cycling of chlorine through the atmosphere, hydrosphere, biosphere, and lithosphere. Chlorine is most commonly found as inorganic chloride ions, or a number of chlorinated organic forms. Over 5,000 biologically-produced chlorinated organics have been identified.

The gold cycle is the biogeochemical cycling of gold through the lithosphere, hydrosphere, atmosphere, and biosphere. Gold is a noble transition metal that is highly mobile in the environment and subject to biogeochemical cycling, driven largely by microorganisms. Gold undergoes processes of solubilization, stabilization, bioreduction, biomineralization, aggregation, and ligand utilization throughout its cycle. These processes are influenced by various microbial populations and cycling of other elements such as carbon, nitrogen, and sulfur. Gold exists in several forms in the Earth's surface environment including Au(I/III)-complexes, nanoparticles, and placer gold particles. The gold biogeochemical cycle is highly complex and strongly intertwined with cycling of other metals including silver, copper, iron, manganese, arsenic, and mercury. Gold is important in the biotech field for applications such as mineral exploration, processing and remediation, development of biosensors and drug delivery systems, industrial catalysts, and for recovery of gold from electronic waste.

The lead cycle is the biogeochemical cycle of lead through the atmosphere, lithosphere, biosphere, and hydrosphere, which has been influenced by anthropogenic activities.

The potassium (K) cycle is the biogeochemical cycle that describes the movement of potassium throughout the Earth's lithosphere, biosphere, atmosphere, and hydrosphere.

The cadmium cycle is a biogeochemical cycle of dispersion and deposition of cadmium through the atmosphere, biosphere, pedosphere, and hydrosphere. Cadmium typically exists in the environment with an oxidation state of +2 but can be found with an oxidation state of +1.
Iodidimonas is a genus of bacteria that oxidizes iodide to iodine. It was isolated from iodide-rich brine associated with natural gas in Kujukuri, Japan.

The manganese cycle is the biogeochemical cycle of manganese through the atmosphere, hydrosphere, biosphere and lithosphere. There are bacteria that oxidise manganese to insoluble oxides, and others that reduce it to Mn2+ in order to use it.

















