
The carbon cycle is that part of the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycle. Carbon is the main component of biological compounds as well as a major component of many rocks such as limestone. The carbon cycle comprises a sequence of events that are key to making Earth capable of sustaining life. It describes the movement of carbon as it is recycled and reused throughout the biosphere, as well as long-term processes of carbon sequestration (storage) to and release from carbon sinks.

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.

A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the carbon cycle, the nitrogen cycle and the water cycle. In each cycle, the chemical element or molecule is transformed and cycled by living organisms and through various geological forms and reservoirs, including the atmosphere, the soil and the oceans. It can be thought of as the pathway by which a chemical substance cycles the biotic compartment and the abiotic compartments of Earth. The biotic compartment is the biosphere and the abiotic compartments are the atmosphere, lithosphere and hydrosphere.

Oxygen cycle refers to the movement of oxygen through the atmosphere (air), biosphere (plants and animals) and the lithosphere (the Earth’s crust). The oxygen cycle demonstrates how free oxygen is made available in each of these regions, as well as how it is used. The oxygen cycle is the biogeochemical cycle of oxygen atoms between different oxidation states in ions, oxides, and molecules through redox reactions within and between the spheres/reservoirs of the planet Earth. The word oxygen in the literature typically refers to the most common oxygen allotrope, elemental/diatomic oxygen (O2), as it is a common product or reactant of many biogeochemical redox reactions within the cycle. Processes within the oxygen cycle are considered to be biological or geological and are evaluated as either a source (O2 production) or sink (O2 consumption).

Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula [CH3Hg]+. It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a bioaccumulative environmental toxicant with a 50-day half-life. Methylmercury is the causative agent of the infamous Minamata disease.
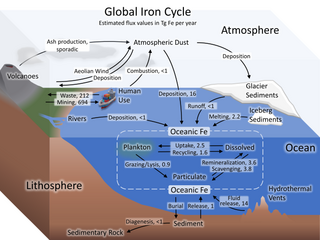
The iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient in primary productivity, and a limiting nutrient in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.

Arctic methane emissions contribute to a rise in methane concentrations in the atmosphere. Whilst the Arctic region is one of many natural sources of the greenhouse gas methane, there is nowadays also a human component to this due to the effects of climate change. In the Arctic, the main human-influenced sources of methane are thawing permafrost, Arctic sea ice melting, clathrate breakdown and Greenland ice sheet melting. This methane release results in a positive climate change feedback, as methane is a powerful greenhouse gas. When permafrost thaws due to global warming, large amounts of organic material can become available for methanogenesis and may therefore be released as methane.
Marine chemistry, also known as ocean chemistry or chemical oceanography, is the study of the chemical composition and processes of the world’s oceans, including the interactions between seawater, the atmosphere, the seafloor, and marine organisms. This field encompasses a wide range of topics, such as the cycling of elements like carbon, nitrogen, and phosphorus, the behavior of trace metals, and the study of gases and nutrients in marine environments. Marine chemistry plays a crucial role in understanding global biogeochemical cycles, ocean circulation, and the effects of human activities, such as pollution and climate change, on oceanic systems. It is influenced by plate tectonics and seafloor spreading, turbidity, currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology.

Mercury regulation in the United States limit the maximum concentrations of mercury (Hg) that is permitted in air, water, soil, food and drugs. The regulations are promulgated by agencies such as the Environmental Protection Agency (EPA) and Food and Drug Administration (FDA), as well as a variety of state and local authorities. EPA published the Mercury and Air Toxics Standards (MATS) regulation in 2012; the first federal standards requiring power plants to limit emissions of mercury and other toxic gases.

The oceanic carbon cycle is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic carbon and organic carbon. Part of the marine carbon cycle transforms carbon between non-living and living matter.

Mercury is a heavy metal that cycles through the atmosphere, water, and soil in various forms to different parts of the world. Due to this natural mercury cycle, irrespective of which part of the world releases mercury it could affect an entirely different part of the world making mercury pollution a global concern. Mercury pollution is now identified as a global problem and awareness has been raised on an international action plan to minimize anthropogenic mercury emissions and clean up mercury pollution. The 2002 Global Mercury Assessment concluded that "International actions to address the global mercury problem should not be delayed". Among many environments that are under the impact of mercury pollution, the ocean is one which cannot be neglected as it has the ability to act as a "storage closet" for mercury. According to a recent model study the total anthropogenic mercury released into the ocean is estimated to be around 80,000 to 45,000 metric tons and two-thirds of this amount is estimated to be found in waters shallower than 1000m level where much consumable fish live. Mercury can bioaccumulate in marine food chains in the form of highly toxic methylmercury which can cause health risks to human seafood consumers. According to statistics, about 66% of global fish consumption comes from the ocean. Therefore, it is important to monitor and regulate oceanic mercury levels to prevent more and more mercury from reaching the human population through seafood consumption.

Marine biogeochemical cycles are biogeochemical cycles that occur within marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. These biogeochemical cycles are the pathways chemical substances and elements move through within the marine environment. In addition, substances and elements can be imported into or exported from the marine environment. These imports and exports can occur as exchanges with the atmosphere above, the ocean floor below, or as runoff from the land.
Caroline Masiello is a biogeochemist who develops tools to better understand the cycling and fate of globally relevant elemental cycles. She is a professor at Rice University in the Department of Earth, Environmental and Planetary Sciences and holds joint appointments in the Chemistry and Biochemistry Departments. Masiello was elected as a Fellow of the Geological Society of America in 2017. She currently leads an interdisciplinary team of scientists who are developing microbial sensors for earth system science.

Aluminum is the third most abundant element in the lithosphere at 82,000 ppm. It occurs in low levels, 0.9 ppm, in humans. Aluminum is known to be an ecotoxicant and expected to be a health risk to people. Global primary production (GPP) of aluminum was about 52 million tons in 2013 and remains one of the world's most important metals. It is used for infrastructure, vehicles, aviation, energy and more due to its lightweight, ductility, and cheap cost. Aluminum is harvested from gibbsite, boehmite, and diaspore which make up bauxite. The aluminum cycle is the biogeochemical cycle by which aluminum is moved through the environment by natural and anthropogenic processes. The biogeochemical cycle of aluminum is integral with silicon and phosphorus. For example, phosphates store aluminum that has been sedimented and aluminum is found in diatoms. Aluminum has been found to prevent growth in organisms by making phosphates less available. The humans/lithosphere ratio (B/L) is very low at 0.000011. This level shows that aluminum is more essential in the lithospheric cycle than in the biotic cycle.

Elsie M. Sunderland is a Canadian toxicologist and environmental scientist and the Gordon McKay Professor of Environmental Chemistry at Harvard University. She studies processes through which human activities increase and modify pollutants in natural ecosystems and living systems.

The boron cycle is the biogeochemical cycle of boron through the atmosphere, lithosphere, biosphere, and hydrosphere.

The arsenic (As) cycle is the biogeochemical cycle of natural and anthropogenic exchanges of arsenic terms through the atmosphere, lithosphere, pedosphere, hydrosphere, and biosphere. Although arsenic is naturally abundant in the Earth's crust, long-term exposure and high concentrations of arsenic can be detrimental to human health.
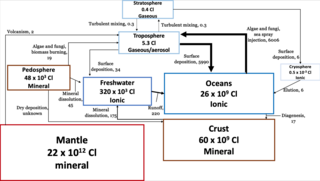
The chlorine cycle (Cl) is the biogeochemical cycling of chlorine through the atmosphere, hydrosphere, biosphere, and lithosphere. Chlorine is most commonly found as inorganic chloride ions, or a number of chlorinated organic forms. Over 5,000 biologically produced chlorinated organics have been identified.

The lead cycle is the biogeochemical cycle of lead through the atmosphere, lithosphere, biosphere, and hydrosphere, which has been influenced by anthropogenic activities.

The fluorine cycle is the series of biogeochemical processes through which fluorine moves through the lithosphere, hydrosphere, atmosphere, and biosphere. Fluorine originates from the Earth’s crust, and its cycling between various sources and sinks is modulated by a variety of natural and anthropogenic processes.



















