
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment or hand-tool methods.

Eutrophication is the process by which an entire body of water, or parts of it, becomes progressively enriched with minerals and nutrients, particularly nitrogen and phosphorus. It has also been defined as "nutrient-induced increase in phytoplankton productivity". Water bodies with very low nutrient levels are termed oligotrophic and those with moderate nutrient levels are termed mesotrophic. Advanced eutrophication may also be referred to as dystrophic and hypertrophic conditions. Eutrophication can affect freshwater or salt water systems. In freshwater ecosystems it is almost always caused by excess phosphorus. In coastal waters on the other hand, the main contributing nutrient is more likely to be nitrogen, or nitrogen and phosphorus together. This depends on the location and other factors.

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.

Water pollution is the contamination of water bodies, usually as a result of human activities, so that it negatively affects its uses. Water bodies include lakes, rivers, oceans, aquifers, reservoirs and groundwater. Water pollution results when contaminants mix with these water bodies. Contaminants can come from one of four main sources: sewage discharges, industrial activities, agricultural activities, and urban runoff including stormwater. Water pollution is either surface water pollution or groundwater pollution. This form of pollution can lead to many problems, such as the degradation of aquatic ecosystems or spreading water-borne diseases when people use polluted water for drinking or irrigation. Another problem is that water pollution reduces the ecosystem services that the water resource would otherwise provide.
The pedosphere is the outermost layer of the Earth that is composed of soil and subject to soil formation processes. It exists at the interface of the lithosphere, atmosphere, hydrosphere and biosphere. The pedosphere is the skin of the Earth and only develops when there is a dynamic interaction between the atmosphere, biosphere, lithosphere and the hydrosphere. The pedosphere is the foundation of terrestrial life on Earth.
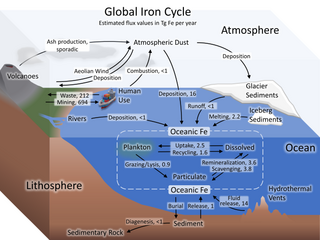
The iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient in primary productivity, and a limiting nutrient in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.
Soil chemistry is the study of the chemical characteristics of soil. Soil chemistry is affected by mineral composition, organic matter and environmental factors. In the early 1870s a consulting chemist to the Royal Agricultural Society in England, named J. Thomas Way, performed many experiments on how soils exchange ions, and is considered the father of soil chemistry. Other scientists who contributed to this branch of ecology include Edmund Ruffin, and Linus Pauling.

Mineral dust is atmospheric aerosol originated from the suspension of minerals constituting the soil, composed of various oxides and carbonates. Human activities lead to 30% of the airborne dust (particulates) load in the atmosphere. The Sahara Desert is the major source of mineral dust, which subsequently spreads across the Mediterranean and Caribbean seas into northern South America, Central America, and eastern North America, and Europe. Additionally, it plays a significant role in the nutrient inflow to the Amazon rainforest. The Gobi Desert is another source of dust in the atmosphere, which affects eastern Asia and western North America.

The phosphorus cycle is the biogeochemical cycle that describes the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the movement of phosphorus, because phosphorus and phosphorus-based compounds are usually solids at the typical ranges of temperature and pressure found on Earth. The production of phosphine gas occurs in only specialized, local conditions. Therefore, the phosphorus cycle should be viewed from whole Earth system and then specifically focused on the cycle in terrestrial and aquatic systems.

Human impact on the nitrogen cycle is diverse. Agricultural and industrial nitrogen (N) inputs to the environment currently exceed inputs from natural N fixation. As a consequence of anthropogenic inputs, the global nitrogen cycle (Fig. 1) has been significantly altered over the past century. Global atmospheric nitrous oxide (N2O) mole fractions have increased from a pre-industrial value of ~270 nmol/mol to ~319 nmol/mol in 2005. Human activities account for over one-third of N2O emissions, most of which are due to the agricultural sector. This article is intended to give a brief review of the history of anthropogenic N inputs, and reported impacts of nitrogen inputs on selected terrestrial and aquatic ecosystems.

Agricultural pollution refers to biotic and abiotic byproducts of farming practices that result in contamination or degradation of the environment and surrounding ecosystems, and/or cause injury to humans and their economic interests. The pollution may come from a variety of sources, ranging from point source water pollution to more diffuse, landscape-level causes, also known as non-point source pollution and air pollution. Once in the environment these pollutants can have both direct effects in surrounding ecosystems, i.e. killing local wildlife or contaminating drinking water, and downstream effects such as dead zones caused by agricultural runoff is concentrated in large water bodies.
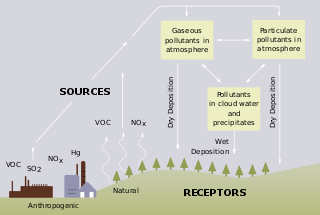
Freshwater acidification occurs when acidic inputs enter a body of fresh water through the weathering of rocks, invasion of acidifying gas, or by the reduction of acid anions, like sulfate and nitrate within a lake. Freshwater acidification is primarily caused by sulfur oxides (SOx) and nitrogen oxides (NOx) entering the water from atmospheric depositions and soil leaching. Carbonic acid and dissolved carbon dioxide can also enter freshwaters, in a similar manner associated with runoff, through carbon dioxide-rich soils. Runoff that contains these compounds may incorporate acidifying hydrogen ions and inorganic aluminum, which can be toxic to marine organisms. Acid rain is also a contributor to freshwater acidification. It is created when SOx and NOx react with water, oxygen, and other oxidants within the clouds.
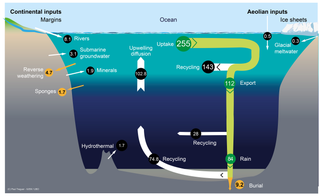
The silica cycle is the biogeochemical cycle in which biogenic silica is transported between the Earth's systems. Silicon is considered a bioessential element and is one of the most abundant elements on Earth. The silica cycle has significant overlap with the carbon cycle and plays an important role in the sequestration of carbon through continental weathering, biogenic export and burial as oozes on geologic timescales.

Saharan dust is an aeolian mineral dust from the Sahara desert, the largest hot desert in the world. The desert spans just over 9 million square kilometers, from the Atlantic Ocean to the Red Sea, from the Mediterranean sea to the Niger River valley and the Sudan region in the south.

The arsenic (As) cycle is the biogeochemical cycle of natural and anthropogenic exchanges of arsenic terms through the atmosphere, lithosphere, pedosphere, hydrosphere, and biosphere. Although arsenic is naturally abundant in the Earth's crust, long-term exposure and high concentrations of arsenic can be detrimental to human health.
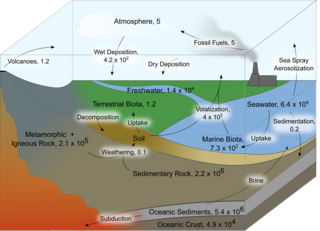
The iodine cycle is a biogeochemical cycle that primarily consists of natural and biological processes that exchange iodine through the lithosphere, hydrosphere, and atmosphere. Iodine exists in many forms, but in the environment, it generally has an oxidation state of -1, 0, or +5.

The lead cycle is the biogeochemical cycle of lead through the atmosphere, lithosphere, biosphere, and hydrosphere, which has been influenced by anthropogenic activities.

The fluorine cycle is the series of biogeochemical processes through which fluorine moves through the lithosphere, hydrosphere, atmosphere, and biosphere. Fluorine originates from the Earth’s crust, and its cycling between various sources and sinks is modulated by a variety of natural and anthropogenic processes.

The manganese cycle is the biogeochemical cycle of manganese through the atmosphere, hydrosphere, biosphere and lithosphere. There are bacteria that oxidise manganese to insoluble oxides, and others that reduce it to Mn2+ in order to use it.

















