
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. Carbon is the main component of biological compounds as well as a major component of many minerals such as limestone. Along with the nitrogen cycle and the water cycle, the carbon cycle comprises a sequence of events that are key to make Earth capable of sustaining life. It describes the movement of carbon as it is recycled and reused throughout the biosphere, as well as long-term processes of carbon sequestration to and release from carbon sinks. Carbon sinks in the land and the ocean each currently take up about one-quarter of anthropogenic carbon emissions each year.

A biogeochemical cycle is the pathway by which a chemical substance cycles the biotic and the abiotic compartments of Earth. The biotic compartment is the biosphere and the abiotic compartments are the atmosphere, hydrosphere and lithosphere. There are biogeochemical cycles for chemical elements, such as for calcium, carbon, hydrogen, mercury, nitrogen, oxygen, phosphorus, selenium, iron and sulfur, as well as molecular cycles, such as for water and silica. There are also macroscopic cycles, such as the rock cycle, and human-induced cycles for synthetic compounds such as polychlorinated biphenyls (PCBs). In some cycles there are reservoirs where a substance can remain or be sequestered for a long period of time.
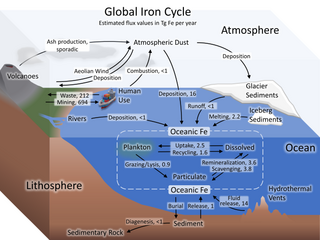
The iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient in primary productivity, and a limiting nutrient in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.

The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration. The global sulfur cycle involves the transformations of sulfur species through different oxidation states, which play an important role in both geological and biological processes. Steps of the sulfur cycle are:
Marine chemistry, also known as ocean chemistry or chemical oceanography, is influenced by plate tectonics and seafloor spreading, turbidity currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology. The field of chemical oceanography studies the chemistry of marine environments including the influences of different variables. Marine life has adapted to the chemistries unique to earth's oceans, and marine ecosystems are sensitive to changes in ocean chemistry.

The mercury cycle is a biogeochemical cycle influenced by natural and anthropogenic processes that transform mercury through multiple chemical forms and environments.

The oceanic carbon cycle is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic carbon and organic carbon. Part of the marine carbon cycle transforms carbon between non-living and living matter.
In Earth science, a geochemical cycle is the pathway that chemical elements take in the surface and crust of the Earth. The term "geochemical" tells us that geological and chemical factors are all included. The migration of heated and compressed chemical elements and compounds such as silicon, aluminium, and general alkali metals through the means of subduction and volcanism is known in the geological world as geochemical cycles.

Marine biogeochemical cycles are biogeochemical cycles that occur within marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. These biogeochemical cycles are the pathways chemical substances and elements move through within the marine environment. In addition, substances and elements can be imported into or exported from the marine environment. These imports and exports can occur as exchanges with the atmosphere above, the ocean floor below, or as runoff from the land.
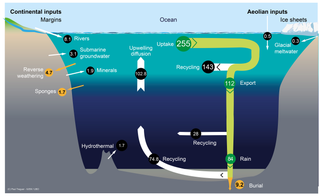
The silica cycle is the biogeochemical cycle in which biogenic silica is transported between the Earth's systems. Silicon is considered a bioessential element and is one of the most abundant elements on Earth. The silica cycle has significant overlap with the carbon cycle and plays an important role in the sequestration of carbon through continental weathering, biogenic export and burial as oozes on geologic timescales.
The aluminum cycle is the biogeochemical cycle by which aluminum is moved through the environment by natural and anthropogenic processes.

The boron cycle is the biogeochemical cycle of boron through the atmosphere, lithosphere, biosphere, and hydrosphere.

The arsenic (As) cycle is the biogeochemical cycle of natural and anthropogenic exchanges of arsenic terms through the atmosphere, lithosphere, pedosphere, hydrosphere, and biosphere. Although arsenic is naturally abundant in the Earth's crust, long-term exposure and high concentrations of arsenic can be detrimental to human health.
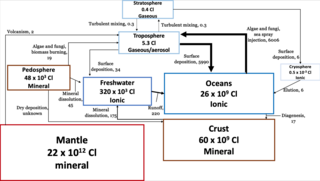
The chlorine cycle (Cl) is the biogeochemical cycling of chlorine through the atmosphere, hydrosphere, biosphere, and lithosphere. Chlorine is most commonly found as inorganic chloride ions, or a number of chlorinated organic forms. Over 5,000 biologically-produced chlorinated organics have been identified.
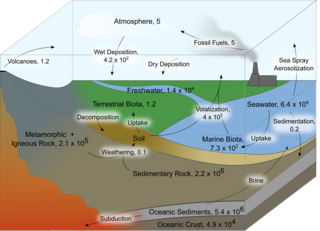
The iodine cycle is a biogeochemical cycle that primarily consists of natural and biological processes that exchange iodine through the lithosphere, hydrosphere, and atmosphere. Iodine exists in many forms, but in the environment, it generally has an oxidation state of -1, 0, or +5.

The lead cycle is the biogeochemical cycle of lead through the atmosphere, lithosphere, biosphere, and hydrosphere, which has been influenced by anthropogenic activities.

The fluorine cycle is the series of biogeochemical processes through which fluorine moves through the lithosphere, hydrosphere, atmosphere, and biosphere. Fluorine originates from the Earth’s crust, and its cycling between various sources and sinks is modulated by a variety of natural and anthropogenic processes.

The global vanadium cycle is controlled by physical and chemical processes that drive the exchange of vanadium between its two main reservoirs: the upper continental crust and the ocean. Anthropogenic processes such as coal and petroleum production release vanadium to the atmosphere.

The cadmium cycle is a biogeochemical cycle of dispersion and deposition of cadmium through the atmosphere, biosphere, pedosphere, and hydrosphere. Cadmium typically exists in the environment with an oxidation state of +2 but can be found with an oxidation state of +1.

The zinc cycle is a biogeochemical cycle that transports zinc through the lithosphere, hydrosphere, and biosphere.
















