Eutrophication is a general term describing a process in which nutrients accumulate in a body of water, resulting in an increased growth of microorganisms that may deplete the water of oxygen. Although eutrophication is a natural process, manmade or cultural eutrophication is far more common and is a rapid process caused by a variety of polluting inputs including poorly treated sewage, industrial wastewater, and fertilizer runoff. Such nutrient pollution usually causes algal blooms and bacterial growth, resulting in the depletion of dissolved oxygen in water and causing substantial environmental degradation.

Cyanobacteria, also called Cyanobacteriota or Cyanophyta, are a phylum of autotrophic gram-negative bacteria that can obtain biological energy via photosynthesis. The name 'cyanobacteria' refers to their color, which similarly forms the basis of cyanobacteria's common name, blue-green algae, although they are not scientifically classified as algae. They appear to have originated in a freshwater or terrestrial environment.
Methanogens are microorganisms that produce methane as a metabolic byproduct in hypoxic conditions. They belong to the domain Archaea and are members of the phylum Euryarchaeota. Methanogens are common in wetlands, where they are responsible for marsh gas, and can occur in the digestive tracts of animals including ruminants and humans, where they are responsible for the methane content of belching and flatulence. In marine sediments, the biological production of methane, termed methanogenesis, is generally confined to where sulfates are depleted below the top layers. Methanogens play an indispensable role in anaerobic wastewater treatments. Other methanogens are extremophiles, found in environments such as hot springs and submarine hydrothermal vents as well as in the "solid" rock of Earth's crust, kilometers below the surface in the deep biosphere.
Methanogenesis or biomethanation is the formation of methane coupled to energy conservation by microbes known as methanogens. Organisms capable of producing methane for energy conservation have been identified only from the domain Archaea, a group phylogenetically distinct from both eukaryotes and bacteria, although many live in close association with anaerobic bacteria. The production of methane is an important and widespread form of microbial metabolism. In anoxic environments, it is the final step in the decomposition of biomass. Methanogenesis is responsible for significant amounts of natural gas accumulations, the remainder being thermogenic.
The purple sulfur bacteria (PSB) are part of a group of Pseudomonadota capable of photosynthesis, collectively referred to as purple bacteria. They are anaerobic or microaerophilic, and are often found in stratified water environments including hot springs, stagnant water bodies, as well as microbial mats in intertidal zones. Unlike plants, algae, and cyanobacteria, purple sulfur bacteria do not use water as their reducing agent, and therefore do not produce oxygen. Instead, they can use sulfur in the form of sulfide, or thiosulfate (as well, some species can use H2, Fe2+, or NO2−) as the electron donor in their photosynthetic pathways. The sulfur is oxidized to produce granules of elemental sulfur. This, in turn, may be oxidized to form sulfuric acid.

Cyanotoxins are toxins produced by cyanobacteria. Cyanobacteria are found almost everywhere, but particularly in lakes and in the ocean where, under high concentration of phosphorus conditions, they reproduce exponentially to form blooms. Blooming cyanobacteria can produce cyanotoxins in such concentrations that they can poison and even kill animals and humans. Cyanotoxins can also accumulate in other animals such as fish and shellfish, and cause poisonings such as shellfish poisoning.

Enteric fermentation is a digestive process by which carbohydrates are broken down by microorganisms into simple molecules for absorption into the bloodstream of an animal. Because of human agricultural reliance in many parts of the world on animals which digest by enteric fermentation, it is the second largest anthropogenic factor for the increase in methane emissions directly after fossil fuel use.

Algal nutrient solutions are made up of a mixture of chemical salts and seawater. Sometimes referred to as "Growth Media", nutrient solutions, provide the materials needed for algae to grow. Nutrient solutions, as opposed to fertilizers, are designed specifically for use in aquatic environments and their composition is much more precise.In a unified system, algal biomass can be collected by utilizing carbon dioxide emanating from power plants and wastewater discharged by both industrial and domestic sources. This approach allows for the concurrent exploitation of the microalgae's capabilities in both carbon dioxide fixation and wastewater treatment.Algae, macroalgae, and microalgae hold promise in addressing critical global challenges. Sustainable development goals can be advanced through algae-based solutions, to promote a healthy global ecosystem.

Human impact on the nitrogen cycle is diverse. Agricultural and industrial nitrogen (N) inputs to the environment currently exceed inputs from natural N fixation. As a consequence of anthropogenic inputs, the global nitrogen cycle (Fig. 1) has been significantly altered over the past century. Global atmospheric nitrous oxide (N2O) mole fractions have increased from a pre-industrial value of ~270 nmol/mol to ~319 nmol/mol in 2005. Human activities account for over one-third of N2O emissions, most of which are due to the agricultural sector. This article is intended to give a brief review of the history of anthropogenic N inputs, and reported impacts of nitrogen inputs on selected terrestrial and aquatic ecosystems.

Algae fuel, algal biofuel, or algal oil is an alternative to liquid fossil fuels that uses algae as its source of energy-rich oils. Also, algae fuels are an alternative to commonly known biofuel sources, such as corn and sugarcane. When made from seaweed (macroalgae) it can be known as seaweed fuel or seaweed oil.

A harmful algal bloom (HAB), or excessive algae growth, is an algal bloom that causes negative impacts to other organisms by production of natural algae-produced toxins, mechanical damage to other organisms, or by other means. HABs are sometimes defined as only those algal blooms that produce toxins, and sometimes as any algal bloom that can result in severely lower oxygen levels in natural waters, killing organisms in marine or fresh waters. Blooms can last from a few days to many months. After the bloom dies, the microbes that decompose the dead algae use up more of the oxygen, generating a "dead zone" which can cause fish die-offs. When these zones cover a large area for an extended period of time, neither fish nor plants are able to survive. Harmful algal blooms in marine environments are often called "red tides".

Arctic methane release is the release of methane from Arctic ocean waters as well as from soils in permafrost regions of the Arctic. While it is a long-term natural process, methane release is exacerbated by global warming. This results in a positive climate change feedback, as methane is a powerful greenhouse gas. The Arctic region is one of many natural sources of methane. Climate change could accelerate methane release in the Arctic, due to the release of methane from existing stores, and from methanogenesis in rotting biomass. When permafrost thaws as a consequence of warming, large amounts of organic material can become available for methanogenesis and may ultimately be released as methane.
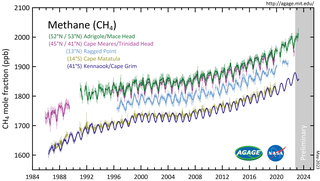
Atmospheric methane is the methane present in Earth's atmosphere. The concentration of atmospheric methane is increasing due to methane emissions, and is causing climate change. Methane is one of the most potent greenhouse gases. Methane's radiative forcing (RF) of climate is direct, and it is the second largest contributor to human-caused climate forcing in the historical period. Methane is a major source of water vapour in the stratosphere through oxidation; and water vapour adds about 15% to methane's radiative forcing effect. The global warming potential (GWP) for methane is about 84 in terms of its impact over a 20-year timeframe, and 28 in terms of its impact over a 100-year timeframe.

The permafrost carbon cycle or Arctic carbon cycle is a sub-cycle of the larger global carbon cycle. Permafrost is defined as subsurface material that remains below 0o C for at least two consecutive years. Because permafrost soils remain frozen for long periods of time, they store large amounts of carbon and other nutrients within their frozen framework during that time. Permafrost represents a large carbon reservoir, one which was often neglected in the initial research determining global terrestrial carbon reservoirs. Since the start of the 2000s, however, far more attention has been paid to the subject, with an enormous growth both in general attention and in the scientific research output.

Greenhouse gas emissions from wetlands of concern consist primarily of methane and nitrous oxide emissions. Wetlands are the largest natural source of atmospheric methane in the world, and are therefore a major area of concern with respect to climate change. Wetlands account for approximately 20–30% of atmospheric methane through emissions from soils and plants, and contribute an approximate average of 161 Tg of methane to the atmosphere per year.

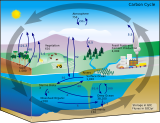
The atmospheric carbon cycle accounts for the exchange of gaseous carbon compounds, primarily carbon dioxide, between Earth's atmosphere, the oceans, and the terrestrial biosphere. It is one of the faster components of the planet's overall carbon cycle, supporting the exchange of more than 200 billion tons of carbon in and out of the atmosphere throughout the course of each year. Atmospheric concentrations of CO2 remain stable over longer timescales only when there exists a balance between these two flows. Methane, Carbon monoxide (CO), and other man-made compounds are present in smaller concentrations and are also part of the atmospheric carbon cycle.
Increasing methane emissions are a major contributor to the rising concentration of greenhouse gases in Earth's atmosphere, and are responsible for up to one-third of near-term global heating. During 2019, about 60% of methane released globally was from human activities, while natural sources contributed about 40%. Reducing methane emissions by capturing and utilizing the gas can produce simultaneous environmental and economic benefits.
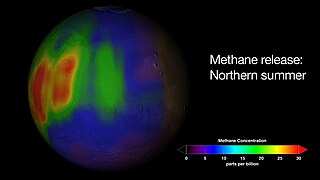
The reported presence of methane in the atmosphere of Mars is of interest to many geologists and astrobiologists, as methane may indicate the presence of microbial life on Mars, or a geochemical process such as volcanism or hydrothermal activity.

Jennifer E. Smith is an American marine ecologist and coral reef expert who works at the Scripps Institution of Oceanography. Her research investigates how physical and biological processes impact the function of marine communities.

Mina Bizic is an environmental microbiologist with particular interest in aquatic systems. She is mostly known for her work on organic matter particles and oxic methane production. She was named a fellow of the Association for the Sciences of Limnology and Oceanography (ASLO) in 2022, and is serving on the ASLO board of directors where she is chairing the Early Career Committee.