A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.
Garlic is a species of bulbous flowering plant in the genus Allium. Its close relatives include the onion, shallot, leek, chive, Welsh onion, and Chinese onion. It is native to Central Asia, South Asia and northeastern Iran. It has long been used as a seasoning and culinary ingredient worldwide, with a history of several thousand years of human consumption and use, including also use as a traditional medicine. It was known to ancient Egyptians and other ancient cultures for which its consumption has had a significant culinary cultural impact, especially across the Mediterranean region and across parts of Asia. It is produced globally but the largest producer is China which produced 73% of the world's supply of garlic in 2021. There are two subspecies and hundreds of varieties of garlic.

Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. It is an isomer of another solvent, butanone.
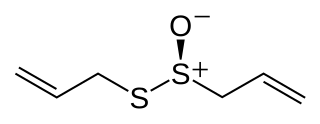
Allicin is an organosulfur compound obtained from garlic and leeks. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. Allicin is unstable and quickly changes into a series of other sulfur-containing compounds such as diallyl disulfide. Allicin is an antifeedant, i.e. the defense mechanism against attacks by pests on the garlic plant.
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring.

Cannabis sativa is an annual herbaceous flowering plant. The species was first classified by Carl Linnaeus in 1753. The specific epithet sativa means 'cultivated'. Indigenous to Eastern Asia, the plant is now of cosmopolitan distribution due to widespread cultivation. It has been cultivated throughout recorded history and used as a source of industrial fiber, seed oil, food, and medicine. It is also used as a recreational drug and for religious and spiritual purposes.

Ajoene is an organosulfur compound found in garlic (Allium sativum) extracts. It is a colorless liquid that contains sulfoxide and disulfide functional groups. The name (and pronunciation) is derived from "ajo", the Spanish word for garlic. It is found as a mixture of up to four stereoisomers, which differ in terms of the stereochemistry of the central alkene (E- vs Z-) and the chirality of the sulfoxide sulfur (R- vs S-).

Ethionine is a non-proteinogenic amino acid structurally related to methionine, with an ethyl group in place of the methyl group.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.
In organic chemistry, the Paal–Knorr synthesis is a reaction used to synthesize substituted furans, pyrroles, or thiophenes from 1,4-diketones. It is a synthetically valuable method for obtaining substituted furans and pyrroles, which are common structural components of many natural products. It was initially reported independently by German chemists Carl Paal and Ludwig Knorr in 1884 as a method for the preparation of furans, and has been adapted for pyrroles and thiophenes. Although the Paal–Knorr synthesis has seen widespread use, the mechanism wasn't fully understood until it was elucidated by V. Amarnath et al. in the 1990s.

Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source of butadiene.

In enzymology, an alliin lyase is an enzyme that catalyzes the chemical reaction

Metalloles are metallacycle derivatives of cyclopentadiene in which the carbon atom at position 5, the saturated carbon, is replaced by a heteroatom. In contrast to its parent compound, the numbering of the metallole starts at the heteroatom. Some of these compounds are described as organometallic compounds, but in the list below quite a number of metalloids are present too. Many metalloles are fluorescent. Polymeric derivatives of pyrrole and thiophene are of interest in molecular electronics. Metalloles, which can also be viewed as structural analogs of pyrrole, include:
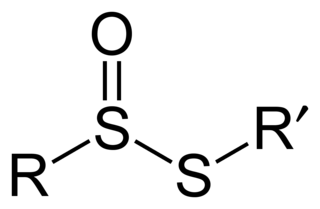
In organosulfur chemistry, thiosulfinate is a functional group consisting of the linkage R-S(O)-S-R. Thiolsulfinates are also named as alkanethiosulfinic acid esters.
Chester J. Cavallito was an American organic chemist. He was particularly known for his work on the chemistry of garlic. Beginning in 1944, with his colleagues, he reported on the isolation from crushed garlic, synthesis and antibiotic activity of a compound he named allicin. Cavallito established that allicin was a member of a class of organosulfur compounds known as thiosulfinates. He also synthesized and reported on the chemical and biological properties of a series of thiosulfinates related to allicin.

Allium is a large genus of monocotyledonous flowering plants with around 1000 different species accepted in botanical science, making Allium the largest genus in the Amaryllidaceae plant family and places Allium amongst the largest plant genera in the world. Many of the species are edible, and some have a long history of cultivation and human consumption as a vegetable including the onion, garlic, scallions, shallots, leeks, and chives, with onions being the second most grown vegetable globally after tomatoes as of 2023.

Eric Block is an American chemist whose research has focused on the chemistry of organosulfur and organoselenium compounds, Allium chemistry, and the chemistry of olfaction. As of 2018, he is Distinguished Professor of Chemistry Emeritus at the University at Albany, SUNY.

Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 receptor agonist which was known as a synthetic homologue of tetrahydrocannabinol (THC), but for the first time in 2019 was isolated as a natural product in trace amounts from Cannabis sativa.

5-Methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile, also known as ROY (red-orange-yellow), is an organic compound which is a chemical intermediate to the drug olanzapine. It has been the subject of intensive study because it can exist in multiple well-characterised crystalline polymorphic forms.