Related Research Articles

Selective estrogen receptor modulators (SERMs), also known as estrogen receptor agonists/antagonists (ERAAs), are a class of drugs that act on estrogen receptors (ERs). Compared to pure ER agonists–antagonists, SERMs are more tissue-specific, allowing them to selectively inhibit or stimulate estrogen-like action in various tissues.
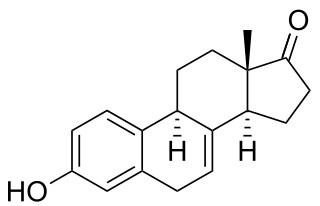
Equilin is a naturally occurring estrogen sex hormone found in horses as well as a medication. It is one of the estrogens present in the estrogen combination drug preparations known as conjugated estrogens and esterified estrogens. CEEs is the most commonly used form of estrogen medications in hormone replacement therapy (HRT) for menopausal symptoms in the United States. Estrone sulfate is the major estrogen in CEEs while equilin sulfate is the second major estrogen in the formulation, present as about 25% of the total.

Tamoxifen, sold under the brand name Nolvadex among others, is a selective estrogen receptor modulator used to prevent breast cancer in women and men. It is also being studied for other types of cancer. It has been used for Albright syndrome. Tamoxifen is typically taken daily by mouth for five years for breast cancer.

Raloxifene, sold under the brand name Evista among others, is a medication used to prevent and treat osteoporosis in postmenopausal women and those on glucocorticoids. For osteoporosis it is less preferred than bisphosphonates. It is also used to reduce the risk of breast cancer in those at high risk. It is taken by mouth.

Bazedoxifene, used as bazedoxifene acetate, is a medication for bone problems and possibly for cancer. It is a third-generation selective estrogen receptor modulator (SERM). Since late 2013 it has had U.S. FDA approval for bazedoxifene as part of the combination drug Duavee in the prevention of postmenopausal osteoporosis. It is also being studied for possible treatment of breast cancer and pancreatic cancer.

Tibolone, sold under the brand name Livial among others, is a medication which is used in menopausal hormone therapy and in the treatment of postmenopausal osteoporosis and endometriosis. The medication is available alone and is not formulated or used in combination with other medications. It is taken by mouth.

Chlorotrianisene (CTA), also known as tri-p-anisylchloroethylene (TACE) and sold under the brand name Tace among others, is a nonsteroidal estrogen related to diethylstilbestrol (DES) which was previously used in the treatment of menopausal symptoms and estrogen deficiency in women and prostate cancer in men, among other indications, but has since been discontinued and is now no longer available. It is taken by mouth.
Hormonal therapy in oncology is hormone therapy for cancer and is one of the major modalities of medical oncology, others being cytotoxic chemotherapy and targeted therapy (biotherapeutics). It involves the manipulation of the endocrine system through exogenous or external administration of specific hormones, particularly steroid hormones, or drugs which inhibit the production or activity of such hormones. Because steroid hormones are powerful drivers of gene expression in certain cancer cells, changing the levels or activity of certain hormones can cause certain cancers to cease growing, or even undergo cell death. Surgical removal of endocrine organs, such as orchiectomy and oophorectomy can also be employed as a form of hormonal therapy.

Selective androgen receptor modulators (SARMs) are a class of drugs that selectively activate the androgen receptor in specific tissues, promoting muscle and bone growth while having less effect on male reproductive tissues like the prostate gland.

Arzoxifene is a selective estrogen receptor modulator (SERM) of the benzothiophene group which was never marketed. It is a potent estrogen antagonist in mammary and uterine tissue while acting as an estrogen agonist to maintain bone density and lower serum cholesterol. Arzoxifene is a highly effective agent for prevention of mammary cancer induced in the rat by the carcinogen nitrosomethylurea and is significantly more potent than raloxifene in this regard. Arzoxifene is devoid of the uterotrophic effects of tamoxifen, suggesting that, in contrast to tamoxifen, it is unlikely that the clinical use of arzoxifene will increase the risk of developing endometrial carcinoma.
Pharmacotoxicology entails the study of the consequences of toxic exposure to pharmaceutical drugs and agents in the health care field. The field of pharmacotoxicology also involves the treatment and prevention of pharmaceutically induced side effects. Pharmacotoxicology can be separated into two different categories: pharmacodynamics, and pharmacokinetics.

Enobosarm, also formerly known as ostarine and by the developmental code names GTx-024, MK-2866, and S-22, is a selective androgen receptor modulator (SARM) which is under development for the treatment of androgen receptor-positive breast cancer in women and for improvement of body composition in people taking GLP-1 receptor agonists like semaglutide. It was also under development for a variety of other indications, including treatment of cachexia, Duchenne muscular dystrophy, muscle atrophy or sarcopenia, and stress urinary incontinence, but development for all other uses has been discontinued. Enobosarm was evaluated for the treatment of muscle wasting related to cancer in late-stage clinical trials, and the drug improved lean body mass in these trials, but it was not effective in improving muscle strength. As a result, enobosarm was not approved and development for this use was terminated. Enobosarm is taken by mouth.

Estetrol (E4), or oestetrol, is one of the four natural estrogenic steroid hormones found in humans, along with estrone (E1), estradiol (E2), and estriol (E3). Estetrol is a major estrogen in the body. In contrast to estrone and estradiol, estetrol is a native estrogen of fetal life. Estetrol is produced exclusively by the fetal liver and is found in detectable levels only during pregnancy, with relatively high levels in the fetus and lower levels in the maternal circulation.

In the field of pharmacology, a selective receptor modulator or SRM is a type of drug that has different effects in different tissues. A SRM may behave as an agonist in some tissues while as an antagonist in others. Hence selective receptor modulators are sometimes referred to as tissue selective drugs or mixed agonists / antagonists. This tissue selective behavior is in contrast to many other drugs that behave either as agonists or antagonists regardless of the tissue in question.
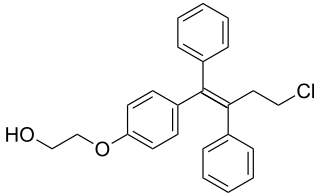
Ospemifene is an oral medication indicated for the treatment of dyspareunia – pain during sexual intercourse – encountered by some women, more often in those who are post-menopausal. Ospemifene is a selective estrogen receptor modulator (SERM) acting similarly to an estrogen on the vaginal epithelium, building vaginal wall thickness which in turn reduces the pain associated with dyspareunia. Dyspareunia is most commonly caused by "vulvar and vaginal atrophy."
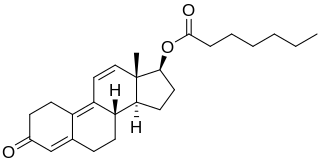
Trenbolone enanthate, known by the nickname Trenabol, is a synthetic and injected anabolic–androgenic steroid (AAS) and a derivative of nandrolone which was never marketed. It is the C17β enanthate ester and a long-acting prodrug of trenbolone. Trenbolone enanthate was never approved for medical or veterinary use but is used in scientific research and has been sold on the internet black market as a designer steroid for bodybuilders and athletes.

A nonsteroidal estrogen is an estrogen with a nonsteroidal chemical structure. The most well-known example is the stilbestrol estrogen diethylstilbestrol (DES). Although nonsteroidal estrogens formerly had an important place in medicine, they have gradually fallen out of favor following the discovery of toxicities associated with high-dose DES starting in the early 1970s, and are now almost never used. On the other hand, virtually all selective estrogen receptor modulators (SERMs) are nonsteroidal, with triphenylethylenes like tamoxifen and clomifene having been derived from DES, and these drugs remain widely used in medicine for the treatment of breast cancer among other indications. In addition to pharmaceutical drugs, many xenoestrogens, including phytoestrogens, mycoestrogens, and synthetic endocrine disruptors like bisphenol A, are nonsteroidal substances with estrogenic activity.

Hexestrol, sold under the brand name Synestrol among others, is a nonsteroidal estrogen which was previously used for estrogen replacement therapy and in the treatment of certain hormone-dependent cancers as well as gynecological disorders but is mostly no longer marketed. It has also been used in the form of esters such as hexestrol diacetate and hexestrol dipropionate. Hexestrol and its esters are taken by mouth, held under the tongue, or via injection into muscle.

Elacestrant, sold under the brand name Orserdu, is a selective estrogen receptor degrader (SERD) used in the treatment of breast cancer. It is taken by mouth.

Bisdehydrodoisynolic acid (BDDA), as the (Z)-isomer ( -BDDA), is a synthetic, nonsteroidal estrogen related to doisynolic acid that was never marketed. It is one of the most potent estrogens known, although it has more recently been characterized as a selective estrogen receptor modulator (SERM). BDDA and other doisynolic acid derivatives display relatively low affinity accompanied by disproportionately high estrogenic potency in vivo, which was eventually determined to be due to transformation into metabolites with greater estrogenic activity. The drug was discovered in 1947 as a degradation product of the reaction of equilenin or dihydroequilenin with potassium hydroxide. It is the seco-analogue of equilenin, while doisynolic acid is the seco-analogue of estrone. These compounds, along with diethylstilbestrol, can be considered to be open-ring analogues of estradiol. The methyl ether of BDDA, doisynoestrol, is also an estrogen, and in contrast to BDDA, has been marketed.
References
- ↑ Zweten (5 September 1997). Antihypertensive Drugs. CRC Press. p. 345. ISBN 9789057021220.
The term "tissue selectivity" is used for an agent showing varying degrees of potency between tissues, with a preferential action in a given one.