Gluten is a structural protein naturally found in certain cereal grains. The term gluten usually refers to the elastic network of a wheat grain's proteins, gliadin and glutenin primarily, that forms readily with the addition of water and often kneading in the case of bread dough. The types of grains that contain gluten include all species of wheat, and barley, rye, and some cultivars of oat; moreover, cross hybrids of any of these cereal grains also contain gluten, e.g. triticale. Gluten makes up 75–85% of the total protein in bread wheat.

Wheat is a grass widely cultivated for its seed, a cereal grain that is a staple food around the world. The many species of wheat together make up the genus Triticum ; the most widely grown is common wheat. The archaeological record suggests that wheat was first cultivated in the regions of the Fertile Crescent around 9600 BC. Botanically, the wheat kernel is a caryopsis, a type of fruit.

Bread is a staple food prepared from a dough of flour and water, usually by baking. Throughout recorded history and around the world, it has been an important part of many cultures' diet. It is one of the oldest human-made foods, having been of significance since the dawn of agriculture, and plays an essential role in both religious rituals and secular culture.

Coeliac disease or celiac disease is a long-term autoimmune disorder, primarily affecting the small intestine, where individuals develop intolerance to gluten, present in foods such as wheat, rye and barley. Classic symptoms include gastrointestinal problems such as chronic diarrhoea, abdominal distention, malabsorption, loss of appetite, and among children failure to grow normally. Non-classic symptoms are more common, especially in people older than two years. There may be mild or absent gastrointestinal symptoms, a wide number of symptoms involving any part of the body, or no obvious symptoms. Coeliac disease was first described in childhood; however, it may develop at any age. It is associated with other autoimmune diseases, such as Type 1 diabetes mellitus and Hashimoto's thyroiditis, among others.

Durum wheat, also called pasta wheat or macaroni wheat, is a tetraploid species of wheat. It is the second most cultivated species of wheat after common wheat, although it represents only 5% to 8% of global wheat production. It was developed by artificial selection of the domesticated emmer wheat strains formerly grown in Central Europe and the Near East around 7000 BC, which developed a naked, free-threshing form. Like emmer, durum wheat is awned. It is the predominant wheat that grows in the Middle East.

A gluten-free diet (GFD) is a nutritional plan that strictly excludes gluten, which is a mixture of prolamin proteins found in wheat, as well as barley, rye, and oats. The inclusion of oats in a gluten-free diet remains controversial, and may depend on the oat cultivar and the frequent cross-contamination with other gluten-containing cereals.

Spelt, also known as dinkel wheat or hulled wheat, is a species of wheat. It is a relict crop, eaten in Central Europe and northern Spain. It is considered a health food since it is high in protein. It is comparable to farro.
Glutenin is a major protein within wheat flour, making up 47% of the total protein content. The glutenins are protein aggregates of high-molecular-mass (HMW) and low-molecular-mass (LMW) subunits with molar masses from about 200,000 to a few million, which are stabilized by intermolecular disulfide bonds, hydrophobic interactions and other forces. Glutenin is responsible for the strength and elasticity of dough.

Gliadin is a class of proteins present in wheat and several other cereals within the grass genus Triticum. Gliadins, which are a component of gluten, are essential for giving bread the ability to rise properly during baking. Gliadins and glutenins are the two main components of the gluten fraction of the wheat seed. This gluten is found in products such as wheat flour. Gluten is split about evenly between the gliadins and glutenins, although there are variations found in different sources.
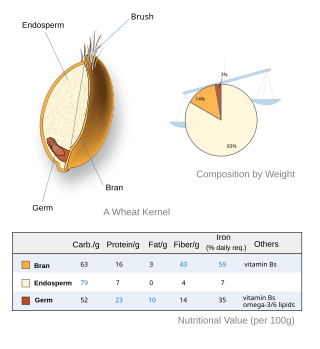
A whole grain is a grain of any cereal and pseudocereal that contains the endosperm, germ, and bran, in contrast to refined grains, which retain only the endosperm.
Hordein is a prolamin glycoprotein, present in barley and some other cereals, together with gliadin and other glycoproteins coming under the general name of gluten. Hordeins are found in the endosperm where one of their functions is to act as a storage unit.
Prolamins are a group of plant storage proteins having a high proline amino acid content. They are found in plants, mainly in the seeds of cereal grains such as wheat (gliadin), barley (hordein), rye (secalin), corn (zein), sorghum (kafirin), and oats (avenin). They are characterised by a high glutamine and proline content, and have poor solubility in water. They solubilise best in strong alcohol (70–80%), light acid, and alkaline solutions. The prolamins of the tribe Triticeae, such as wheat gliadin, and related proteins are known to trigger coeliac disease, an autoimmune condition, in genetically predisposed individuals.

Wheat allergy is an allergy to wheat which typically presents itself as a food allergy, but can also be a contact allergy resulting from occupational exposure. Like all allergies, wheat allergy involves immunoglobulin E and mast cell response. Typically the allergy is limited to the seed storage proteins of wheat. Some reactions are restricted to wheat proteins, while others can react across many varieties of seeds and other plant tissues. Wheat allergy is rare. Prevalence in adults was estimated to be 0.21% in a 2012 study in Japan.

Triticeae is a botanical tribe within the subfamily Pooideae of grasses that includes genera with many domesticated species. Major crop genera found in this tribe include wheat, barley, and rye; crops in other genera include some for human consumption, and others used for animal feed or rangeland protection. Among the world's cultivated species, this tribe has some of the most complex genetic histories. An example is bread wheat, which contains the genomes of three species with only one being a wheat Triticum species. Seed storage proteins in the Triticeae are implicated in various food allergies and intolerances.

Gluten-related disorders is the term for the diseases triggered by gluten, including celiac disease (CD), non-celiac gluten sensitivity (NCGS), gluten ataxia, dermatitis herpetiformis (DH) and wheat allergy. The umbrella category has also been referred to as gluten intolerance, though a multi-disciplinary physician-led study, based in part on the 2011 International Coeliac Disease Symposium, concluded that the use of this term should be avoided due to a lack of specificity.
Anti-gliadin antibodies are produced in response to gliadin, a prolamin found in wheat. In bread wheat it is encoded by three different alleles, AA, BB, and DD. These alleles can produce slightly different gliadins, which can cause the body to produce different antibodies. Some of these antibodies can detect proteins in specific grass taxa such as Triticeae, while others react sporadically with certain species in those taxa, or over many taxonomically defined grass tribes.
Glutelins are a class of prolamin proteins found in the endosperm of certain seeds of the grass family. They constitute a major component of the protein composite collectively referred to as gluten. Glutenin is the most common glutelin, as it is found in wheat and is responsible for some of the refined baking properties in bread wheat. The glutelins of barley and rye have also been identified. Glutelins are the primary protein form of energy storage in the endosperm of rice grains.
Oat sensitivity represents a sensitivity to the proteins found in oats, Avena sativa. Sensitivity to oats can manifest as a result of allergy to oat seed storage proteins either inhaled or ingested. A more complex condition affects individuals who have gluten-sensitive enteropathy in which there is an autoimmune response to avenin, the glutinous protein in oats similar to the gluten within wheat. Sensitivity to oat foods can also result from their frequent contamination by wheat, barley, or rye particles.
The immunochemistry of Triticeae glutens is important in several inflammatory diseases. It can be subdivided into innate responses, class II mediated presentation, class I mediated stimulation of killer cells, and antibody recognition. The responses to gluten proteins and polypeptide regions differs according to the type of gluten sensitivity. The response is also dependent on the genetic makeup of the human leukocyte antigen genes. In gluten sensitive enteropathy, there are four types of recognition, innate immunity, HLA-DQ, and antibody recognition of gliadin and transglutaminase. With idiopathic gluten sensitivity only antibody recognition to gliadin has been resolved. In wheat allergy, the response pathways are mediated through IgE against other wheat proteins and other forms of gliadin.

The cupin superfamily is a diverse superfamily of proteins named after its conserved barrel domain. The superfamily includes a wide variety of enzymes as well as non-enzymatic seed storage proteins.