Related Research Articles

Microvilli are microscopic cellular membrane protrusions that increase the surface area for diffusion and minimize any increase in volume, and are involved in a wide variety of functions, including absorption, secretion, cellular adhesion, and mechanotransduction.

Intestinal villi are small, finger-like projections that extend into the lumen of the small intestine. Each villus is approximately 0.5–1.6 mm in length, and has many microvilli projecting from the enterocytes of its epithelium which collectively form the striated or brush border. Each of these microvilli are about 1 µm in length, around 1000 times shorter than a single villus. The intestinal villi are much smaller than any of the circular folds in the intestine.

Enterocytes, or intestinal absorptive cells, are simple columnar epithelial cells which line the inner surface of the small and large intestines. A glycocalyx surface coat contains digestive enzymes. Microvilli on the apical surface increase its surface area. This facilitates transport of numerous small molecules into the enterocyte from the intestinal lumen. These include broken down proteins, fats, and sugars, as well as water, electrolytes, vitamins, and bile salts. Enterocytes also have an endocrine role, secreting hormones such as leptin.
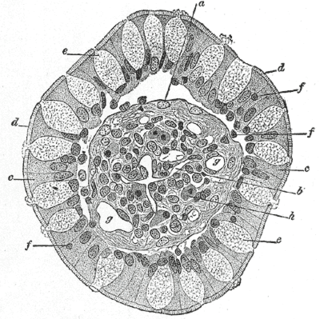
Goblet cells are simple columnar epithelial cells that secrete gel-forming mucins, like mucin 5AC. The goblet cells mainly use the merocrine method of secretion, secreting vesicles into a duct, but may use apocrine methods, budding off their secretions, when under stress. The term goblet refers to the cell's goblet-like shape. The apical portion is shaped like a cup, as it is distended by abundant mucus laden granules; its basal portion lacks these granules and is shaped like a stem.
Gut-associated lymphoid tissue (GALT) is a component of the mucosa-associated lymphoid tissue (MALT) which works in the immune system to protect the body from invasion in the gut.
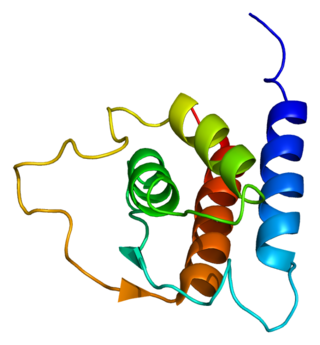
Interleukin 13 (IL-13) is a protein that in humans is encoded by the IL13 gene. IL-13 was first cloned in 1993 and is located on chromosome 5q31.1 with a length of 1.4kb. It has a mass of 13 kDa and folds into 4 alpha helical bundles. The secondary structural features of IL-13 are similar to that of Interleukin 4 (IL-4); however it only has 25% sequence identity to IL-4 and is capable of IL-4 independent signaling. IL-13 is a cytokine secreted by T helper type 2 (Th2) cells, CD4 cells, natural killer T cell, mast cells, basophils, eosinophils and nuocytes. Interleukin-13 is a central regulator in IgE synthesis, goblet cell hyperplasia, mucus hypersecretion, airway hyperresponsiveness, fibrosis and chitinase up-regulation. It is a mediator of allergic inflammation and different diseases including asthma.

Paneth cells are cells in the small intestine epithelium, alongside goblet cells, enterocytes, and enteroendocrine cells. Some can also be found in the cecum and appendix. They are located below the intestinal stem cells in the intestinal glands and the large eosinophilic refractile granules that occupy most of their cytoplasm.
Intestinal permeability is a term describing the control of material passing from inside the gastrointestinal tract through the cells lining the gut wall, into the rest of the body. The intestine normally exhibits some permeability, which allows nutrients to pass through the gut, while also maintaining a barrier function to keep potentially harmful substances from leaving the intestine and migrating to the body more widely. In a healthy human intestine, small particles can migrate through tight junction claudin pore pathways, and particles up to 10–15 Å can transit through the paracellular space uptake route. There is some evidence abnormally increased intestinal permeability may play a role in some chronic diseases and inflammatory conditions. The most well understood condition with observed increased intestinal permeability is celiac disease.

In histology, an intestinal gland is a gland found in between villi in the intestinal epithelium lining of the small intestine and large intestine. The glands and intestinal villi are covered by epithelium, which contains multiple types of cells: enterocytes, goblet cells, enteroendocrine cells, cup cells, tuft cells, and at the base of the gland, Paneth cells and stem cells.

Respiratory epithelium, or airway epithelium, is a type of ciliated columnar epithelium found lining most of the respiratory tract as respiratory mucosa, where it serves to moisten and protect the airways. It is not present in the vocal cords of the larynx, or the oropharynx and laryngopharynx, where instead the epithelium is stratified squamous. It also functions as a barrier to potential pathogens and foreign particles, preventing infection and tissue injury by the secretion of mucus and the action of mucociliary clearance.
Microfold cells are found in the gut-associated lymphoid tissue (GALT) of the Peyer's patches in the small intestine, and in the mucosa-associated lymphoid tissue (MALT) of other parts of the gastrointestinal tract. These cells are known to initiate mucosal immunity responses on the apical membrane of the M cells and allow for transport of microbes and particles across the epithelial cell layer from the gut lumen to the lamina propria where interactions with immune cells can take place.
Intraepithelial lymphocytes (IEL) are lymphocytes found in the epithelial layer of mammalian mucosal linings, such as the gastrointestinal (GI) tract and reproductive tract. However, unlike other T cells, IELs do not need priming. Upon encountering antigens, they immediately release cytokines and cause killing of infected target cells. In the GI tract, they are components of gut-associated lymphoid tissue (GALT).

A taste receptor or tastant is a type of cellular receptor which facilitates the sensation of taste. When food or other substances enter the mouth, molecules interact with saliva and are bound to taste receptors in the oral cavity and other locations. Molecules which give a sensation of taste are considered "sapid".

The intestinal epithelium is the single cell layer that form the luminal surface (lining) of both the small and large intestine (colon) of the gastrointestinal tract. Composed of simple columnar epithelial cells, it serves two main functions: absorbing useful substances into the body and restricting the entry of harmful substances. As part of its protective role, the intestinal epithelium forms an important component of the intestinal mucosal barrier. Certain diseases and conditions are caused by functional defects in the intestinal epithelium. On the other hand, various diseases and conditions can lead to its dysfunction which, in turn, can lead to further complications.

Mucosal immunology is the study of immune system responses that occur at mucosal membranes of the intestines, the urogenital tract, and the respiratory system. The mucous membranes are in constant contact with microorganisms, food, and inhaled antigens. In healthy states, the mucosal immune system protects the organism against infectious pathogens and maintains a tolerance towards non-harmful commensal microbes and benign environmental substances. Disruption of this balance between tolerance and deprivation of pathogens can lead to pathological conditions such as food allergies, irritable bowel syndrome, susceptibility to infections, and more.
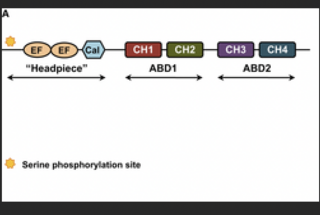
Plastin is part of a family of actin-bundling proteins, specifically the α-actinin family of actin-binding protein, which are found in many lifeforms, from humans and other animals to plants and yeasts. These proteins are known to cross-link actin filaments into bundles for various cell purposes.
Innate lymphoid cells (ILCs) are the most recently discovered family of innate immune cells, derived from common lymphoid progenitors (CLPs). In response to pathogenic tissue damage, ILCs contribute to immunity via the secretion of signalling molecules, and the regulation of both innate and adaptive immune cells. ILCs are primarily tissue resident cells, found in both lymphoid, and non- lymphoid tissues, and rarely in the blood. They are particularly abundant at mucosal surfaces, playing a key role in mucosal immunity and homeostasis. Characteristics allowing their differentiation from other immune cells include the regular lymphoid morphology, absence of rearranged antigen receptors found on T cells and B cells, and phenotypic markers usually present on myeloid or dendritic cells.

The intestinal mucosal barrier, also referred to as intestinal barrier, refers to the property of the intestinal mucosa that ensures adequate containment of undesirable luminal contents within the intestine while preserving the ability to absorb nutrients. The separation it provides between the body and the gut prevents the uncontrolled translocation of luminal contents into the body proper. Its role in protecting the mucosal tissues and circulatory system from exposure to pro-inflammatory molecules, such as microorganisms, toxins, and antigens is vital for the maintenance of health and well-being. Intestinal mucosal barrier dysfunction has been implicated in numerous health conditions such as: food allergies, microbial infections, irritable bowel syndrome, inflammatory bowel disease, celiac disease, metabolic syndrome, non-alcoholic fatty liver disease, diabetes, and septic shock.
Airway basal cells are found deep in the respiratory epithelium, attached to, and lining the basement membrane.

De’Broski. R. Herbert is an immunologist, parasitologist, academic, and biomedical researcher. He is currently Full Professor of Immunology, and Penn Presidential Professor at the University of Pennsylvania School of Veterinary Medicine. He is also the Associate Director for Institute of Infectious and Zoonotic Disease (PennVet), and an affiliated Scientist at the Monell Chemical Senses Center.
References
- 1 2 Gerbe F, Jay P (November 2016). "Intestinal tuft cells: epithelial sentinels linking luminal cues to the immune system" (PDF). Mucosal Immunology. 9 (6): 1353–1359. doi: 10.1038/mi.2016.68 . PMID 27554294.
- ↑ Leslie M (March 28, 2019). "Closing in on a century-old mystery, scientists are figuring out what the body's 'tuft cells' do". Science . doi:10.1126/science.aax4947. S2CID 193049740.
- ↑ Harris N (March 2016). "IMMUNOLOGY. The enigmatic tuft cell in immunity". Science. 351 (6279): 1264–5. Bibcode:2016Sci...351.1264H. doi:10.1126/science.aaf5215. PMID 26989236. S2CID 206648737.
- ↑ Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, et al. (March 2016). "Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut". Science. 351 (6279): 1329–33. Bibcode:2016Sci...351.1329H. doi:10.1126/science.aaf1648. PMC 5528851 . PMID 26847546.
- 1 2 3 4 Gerbe F, Legraverend C, Jay P (September 2012). "The intestinal epithelium tuft cells: specification and function". Cellular and Molecular Life Sciences. 69 (17): 2907–17. doi:10.1007/s00018-012-0984-7. PMC 3417095 . PMID 22527717.
- ↑ O'Donnell, Anne Marie; Nakamura, Hiroki; Puri, Prem (2019-11-10). ""Tuft Cells": A New Player in Hirschsprung's Disease". European Journal of Pediatric Surgery. 30 (1): s–0039–1700549. doi:10.1055/s-0039-1700549. ISSN 0939-7248. PMID 31707728. S2CID 207936025.
- 1 2 3 Nevo S, Kadouri N, Abramson J (June 2019). "Tuft cells: From the mucosa to the thymus". Immunology Letters. 210: 1–9. doi:10.1016/j.imlet.2019.02.003. PMID 30904566. S2CID 85501296.
- 1 2 3 4 von Moltke J (2018). "Intestinal Tuft Cells". Physiology of the Gastrointestinal Tract. Elsevier. pp. 721–733. doi:10.1016/b978-0-12-809954-4.00031-1. ISBN 978-0-12-809954-4 . Retrieved 2020-01-29.
- 1 2 Banerjee A, McKinley ET, von Moltke J, Coffey RJ, Lau KS (May 2018). "Interpreting heterogeneity in intestinal tuft cell structure and function". The Journal of Clinical Investigation. 128 (5): 1711–1719. doi:10.1172/JCI120330. PMC 5919882 . PMID 29714721.
- ↑ Reid L, Meyrick B, Antony VB, Chang LY, Crapo JD, Reynolds HY (July 2005). "The mysterious pulmonary brush cell: a cell in search of a function". American Journal of Respiratory and Critical Care Medicine. 172 (1): 136–9. doi:10.1164/rccm.200502-203WS. PMC 2718446 . PMID 15817800.
- ↑ Banerjee A, Herring CA, Simmons AJ, Kim H, McKinley ET, Chen B, et al. (May 2019). "526 – The Role of Tuft Cell Specification and Function in Inflammatory Ileitis". Gastroenterology. 156 (6): S–106. doi: 10.1016/s0016-5085(19)37056-8 .
- ↑ Steele SP, Melchor SJ, Petri WA (November 2016). "Tuft Cells: New Players in Colitis". Trends in Molecular Medicine. 22 (11): 921–924. doi:10.1016/j.molmed.2016.09.005. PMC 5159242 . PMID 27717671.