
Integrins are transmembrane receptors that help cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface.

A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents.
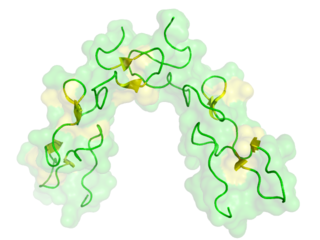
Disintegrins are a family of small proteins from viper venoms that function as potent inhibitors of both platelet aggregation and integrin-dependent cell adhesion.

ICAM-1 also known as CD54 is a protein that in humans is encoded by the ICAM1 gene. This gene encodes a cell surface glycoprotein which is typically expressed on endothelial cells and cells of the immune system. It binds to integrins of type CD11a / CD18, or CD11b / CD18 and is also exploited by rhinovirus as a receptor for entry into respiratory epithelium.

CD11c, also known as Integrin, alpha X (ITGAX), is a gene that encodes for CD11c.
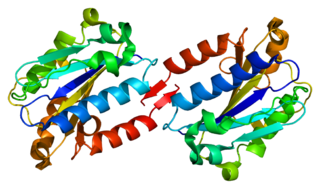
Integrin, alpha L , also known as ITGAL, is a protein that in humans is encoded by the ITGAL gene. CD11a functions in the immune system. It is involved in cellular adhesion and costimulatory signaling. It is the target of the drug efalizumab.

Integrin alpha M (ITGAM) is one protein subunit that forms heterodimeric integrin alpha-M beta-2 (αMβ2) molecule, also known as macrophage-1 antigen (Mac-1) or complement receptor 3 (CR3). ITGAM is also known as CR3A, and cluster of differentiation molecule 11B (CD11B). The second chain of αMβ2 is the common integrin β2 subunit known as CD18, and integrin αMβ2 thus belongs to the β2 subfamily integrins.
Lymphocyte function-associated antigen 1 (LFA-1) is an integrin found on lymphocytes and other leukocytes. LFA-1 plays a key role in emigration, which is the process by which leukocytes leave the bloodstream to enter the tissues. LFA-1 also mediates firm arrest of leukocytes. Additionally, LFA-1 is involved in the process of cytotoxic T cell mediated killing as well as antibody mediated killing by granulocytes and monocytes. As of 2007, LFA-1 has 6 known ligands: ICAM-1, ICAM-2, ICAM-3, ICAM-4, ICAM-5, and JAM-A. LFA-1/ICAM-1 interactions have recently been shown to stimulate signaling pathways that influence T cell differentiation. LFA-1 belongs to the integrin superfamily of adhesion molecules.

In molecular biology, CD18 is an integrin beta chain protein that is encoded by the ITGB2 gene in humans. Upon binding with one of a number of alpha chains, CD18 is capable of forming multiple heterodimers, which play significant roles in cellular adhesion and cell surface signaling, as well as important roles in immune responses. CD18 also exists in soluble, ligand binding forms. Deficiencies in CD18 expression can lead to adhesion defects in circulating white blood cells in humans, reducing the immune system's ability to fight off foreign invaders.
Platelet membrane glycoproteins are surface glycoproteins found on platelets (thrombocytes) which play a key role in hemostasis. When the blood vessel wall is damaged, platelet membrane glycoproteins interact with the extracellular matrix.

Collagen alpha-1(VI) chain is a protein that in humans is encoded by the COL6A1 gene.

Glycoprotein Ib (platelet), beta polypeptide (GP1BB) also known as CD42c, is a protein that in humans is encoded by the GP1BB gene.

Collagen alpha-3(VI) chain is a protein that in humans is encoded by the COL6A3 gene. This protein is an alpha chain of type VI collagen that aids in microfibril formation. As part of type VI collagen, this protein has been implicated in Bethlem myopathy, Ullrich congenital muscular dystrophy (UCMD), and other diseases related to muscle and connective tissue.

Matrilin-3 is a protein that in humans is encoded by the MATN3 gene. It is linked to the development of many types of cartilage, and part of the Matrilin family, which includes Matrilin-1, Matrilin-2, Matrilin-3, and Matrilin-4, a family of filamentous-forming adapter oligomeric extracellular proteins that are linked to the formation of cartilage and bone, as well as maintaining homeostasis after development. It is considered an extracellular matrix protein that functions as an adapter protein where the Matrilin-3 subunit can form both homo-tetramers and hetero-oligomers with subunits from Matrilin-1 which is the cartilage matrix protein. This restricted tissue has been strongly expressed in growing skeletal tissue as well as cartilage and bone.

Integrin alpha-11 is a protein that, in humans, is encoded by the ITGA11 gene.
The following outline is provided as an overview of and topical guide to immunology:
Von Willebrand factor, type C is a protein domain is found in various blood plasma proteins: complement factors B, C2, CR3 and CR4; the integrins (I-domains); collagen types VI, VII, XII and XIV; and other extracellular proteins.

In molecular biology, trimeric autotransporter adhesins (TAAs), are proteins found on the outer membrane of Gram-negative bacteria. Bacteria use TAAs in order to infect their host cells via a process called cell adhesion. TAAs also go by another name, oligomeric coiled-coil adhesins, which is shortened to OCAs. In essence, they are virulence factors, factors that make the bacteria harmful and infective to the host organism.
Von Willebrand factor type D domain (vWD) is an evolutionarily-conserved protein domain found in, among others, the von Willebrand factor (vWF). vWF is a large multimeric glycoprotein and it is synthesized by a type of bone marrow cell called megakaryocytes. The vWD domain allows vWF to perform its blood-clotting function by carrying factor VIII around.

Collagen α-1 (XXIII) chain is a protein encoded by COL23A1 gene, which is located on chromosome 5q35 in humans, and on chromosome 11B1+2 in mice. The location of this gene was discovered by genomic sequence analysis.















