Calreticulin also known as calregulin, CRP55, CaBP3, calsequestrin-like protein, and endoplasmic reticulum resident protein 60 (ERp60) is a protein that in humans is encoded by the CALR gene.

The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae, and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa.
Antigen processing, or the cytosolic pathway, is an immunological process that prepares antigens for presentation to special cells of the immune system called T lymphocytes. It is considered to be a stage of antigen presentation pathways. This process involves two distinct pathways for processing of antigens from an organism's own (self) proteins or intracellular pathogens, or from phagocytosed pathogens ; subsequent presentation of these antigens on class I or class II major histocompatibility complex (MHC) molecules is dependent on which pathway is used. Both MHC class I and II are required to bind antigens before they are stably expressed on a cell surface. MHC I antigen presentation typically involves the endogenous pathway of antigen processing, and MHC II antigen presentation involves the exogenous pathway of antigen processing. Cross-presentation involves parts of the exogenous and the endogenous pathways but ultimately involves the latter portion of the endogenous pathway.

MHC class I molecules are one of two primary classes of major histocompatibility complex (MHC) molecules and are found on the cell surface of all nucleated cells in the bodies of vertebrates. They also occur on platelets, but not on red blood cells. Their function is to display peptide fragments of proteins from within the cell to cytotoxic T cells; this will trigger an immediate response from the immune system against a particular non-self antigen displayed with the help of an MHC class I protein. Because MHC class I molecules present peptides derived from cytosolic proteins, the pathway of MHC class I presentation is often called cytosolic or endogenous pathway.
The terms glycans and polysaccharides are defined by IUPAC as synonyms meaning "compounds consisting of a large number of monosaccharides linked glycosidically". However, in practice the term glycan may also be used to refer to the carbohydrate portion of a glycoconjugate, such as a glycoprotein, glycolipid, or a proteoglycan, even if the carbohydrate is only an oligosaccharide. Glycans usually consist solely of O-glycosidic linkages of monosaccharides. For example, cellulose is a glycan composed of β-1,4-linked D-glucose, and chitin is a glycan composed of β-1,4-linked N-acetyl-D-glucosamine. Glycans can be homo- or heteropolymers of monosaccharide residues, and can be linear or branched.

Endoplasmic-reticulum-associated protein degradation (ERAD) designates a cellular pathway which targets misfolded proteins of the endoplasmic reticulum for ubiquitination and subsequent degradation by a protein-degrading complex, called the proteasome.
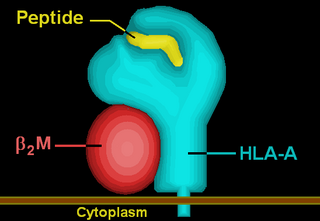
HLA-A is a group of human leukocyte antigens (HLA) that are encoded by the HLA-A locus, which is located at human chromosome 6p21.3. HLA is a major histocompatibility complex (MHC) antigen specific to humans. HLA-A is one of three major types of human MHC class I transmembrane proteins. The others are HLA-B and HLA-C. The protein is a heterodimer, and is composed of a heavy α chain and smaller β chain. The α chain is encoded by a variant HLA-A gene, and the β chain (β2-microglobulin) is an invariant β2 microglobulin molecule. The β2 microglobulin protein is encoded by the B2M gene, which is located at chromosome 15q21.1 in humans.
The unfolded protein response (UPR) is a cellular stress response related to the endoplasmic reticulum (ER) stress. It has been found to be conserved between mammalian species, as well as yeast and worm organisms.

TAP-associated glycoprotein, also known as tapasin or TAPBP, is a protein that in humans is encoded by the TAPBP gene.

Protein disulfide-isomerase A3 (PDIA3), also known as glucose-regulated protein, 58-kD (GRP58), is an isomerase enzyme. This protein localizes to the endoplasmic reticulum (ER) and interacts with lectin chaperones calreticulin and calnexin (CNX) to modulate folding of newly synthesized glycoproteins. It is thought that complexes of lectins and this protein mediate protein folding by promoting formation of disulfide bonds in their glycoprotein substrates.

Heat shock protein 90kDa beta member 1 (HSP90B1), known also as endoplasmin, gp96, grp94, or ERp99, is a chaperone protein that in humans is encoded by the HSP90B1 gene.

Peptidyl-prolyl cis-trans isomerase B is an enzyme that is encoded by the PPIB gene. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to regulate protein folding of type I collagen. Generally, PPIases are found in all eubacteria and eukaryotes, as well as in a few archaebacteria, and thus are highly conserved.

Activating transcription factor 6, also known as ATF6, is a protein that, in humans, is encoded by the ATF6 gene and is involved in the unfolded protein response.

Growth arrest-specific protein 3 (GAS-3), also called peripheral myelin protein 22 (PMP22), is a protein which in humans is encoded by the PMP22 gene.

Binding immunoglobulin protein (BiPS) also known as 78 kDa glucose-regulated protein (GRP-78) or heat shock 70 kDa protein 5 (HSPA5) is a protein that in humans is encoded by the HSPA5 gene.
UGGT, or UDP-glucose:glycoprotein glucosyltransferase, is a soluble enzyme resident in the lumen of the endoplasmic reticulum (ER).

In molecular biology, the calreticulin protein family is a family of calcium-binding proteins. This family includes Calreticulin, Calnexin and Camlegin.

Protein disulfide isomerase family A member 2 is a protein that in humans is encoded by the PDIA2 gene.

The peptide-loading complex (PLC) is a short-lived, multisubunit membrane protein complex that is located in the endoplasmic reticulum (ER). It orchestrates peptide translocation and selection by major histocompatibility complex class I (MHC-I) molecules. Stable peptide-MHC I complexes are released to the cell surface to promote T-cell response against malignant or infected cells. In turn, T-cells recognize the activated peptides, which could be immunogenic or non-immunogenic.
John J. M. Bergeron, is a Canadian cell biologist and biochemist. He is an Emeritus Robert Reford Professor of Anatomy and Professor of Medicine at McGill University in Montreal, Quebec, Canada. He is a Rhodes Scholar. He is best known for the discovery of calnexin, endosomal signalling and organellar proteomics.