Forkhead box protein L2 is a protein that in humans is encoded by the FOXL2 gene. [5] [6]
Forkhead box protein L2 is a protein that in humans is encoded by the FOXL2 gene. [5] [6]
FOXL2 (OMIM 605597) is a transcription factor belonging to the forkhead box (FOX) superfamily, characterized by the forkhead box/winged-helix DNA-binding domain. FOXL2 plays an important role in ovarian development and function. [6] In postnatal ovaries FOXL2 regulates granulosa cell differentiation and supports the growth of the pre-ovulatory follicles during adult life. [7] In addition, the FOXL2 protein will prevent the formation of testes by suppressing expression of SOX9. [8] In mice, FOXL2 is also expressed in pituitary cells [9] where it is required for FSH expression. [10]
FOXL2 has several post-translational modifications that modulate its stability, subcellular localization and pro-apoptotic activity. [11] By a yeast-two-hybrid screening, 10 novel protein partners of FOXL2 were discovered. The interactions were confirmed by co-immunoprecipitation experiments between FOXL2 and CXXC4 (IDAX), CXXC5 (RINF/WID), CREM, GMEB1 (P96PIF), NR2C1 (TR2), SP100, RPLP1, BAF (BANF1), XRCC6 (KU70) and SIRT1. [12]
FOXL2 is involved in sex determination. FOXL2 knockout in mature mouse ovaries appears to cause the ovary's somatic cells to transdifferentiate to the equivalent cell types ordinarily found in the testes. [13] Polled Intersex Syndrome in goats is caused by a biallelic loss-of-function in FOXL2 transcription and leads to in utero female-to-male sex-reversal. [14]
Several SNPs (Single Variant Polymorphisms) in the genomic region 3q23 overlapping the forkhead box L2 (FOXL2) were found associated with eyebrow thickness. In Europeans, East Asians, and South Asians, the derived allele is above ~90% frequency, and in Africans, it is above ~75%. Native Americans, particularly Peruvians, have a relatively high frequency of the homozygous ancestral allele, which significantly decreases eyebrow thickness. All primates and archaic humans share the ancestral allele. [15]
Mutations in this gene are a cause of blepharophimosis, ptosis, epicanthus inversus syndrome and/or premature ovarian failure (POF) 3. [6] Predicting the occurrence of POF based on the nature of the missense mutations in FOXL2 was a medical challenge. However, a correlation between the transcriptional activity of FOXL2 variants and the type of BPES was found. [16] Moreover, by studying the effects of natural and artificial mutations in the forkhead domain of FOXL2, a clear correlation between the orientation of amino-acid side chains in the DNA-binding domain and transcriptional activity is founded, providing the first (in silico) predictive tool of the effects of FOXL2 missense mutations. [17]
A missense mutation in the FOXL2 gene, C134W, is typically found in adult granulosa cell tumors but not in other ovarian cancers nor in juvenile granulosa cell tumors. [7]
In addition to ovarian expression of FOXL2, there have been recent studies to suggest that overexpression of FOXL2 has been implicated in endometriosis in addition to activin A. [18]
One study has found that FOXL2 is required for SF-1-induced ovarian AMH regulation by interactions between FOXL2 protein and SF-1; a mutated FOXL2 could not interact with SF-1 normally and thus could not regulate ovarian AMH as normal. [19]
In a knockout study in mice, the granulosa cells of the ovaries failed to undergo the squamous-to-cuboidal transition, which led to the arrest of folliculogenesis. [20]

Granulosa cell tumours are tumours that arise from granulosa cells. They are estrogen secreting tumours and present as large, complex, ovarian masses. These tumours are part of the sex cord–gonadal stromal tumour or non-epithelial group of tumours. Although granulosa cells normally occur only in the ovary, granulosa cell tumours occur in both ovaries and testicles. These tumours should be considered malignant and treated in the same way as other malignant tumours of ovary. The ovarian disease has two forms, juvenile and adult, both characterized by indolent growth, and therefore has high recovery rates. The staging system for these tumours is the same as for epithelial tumours and most present as stage I. The peak age at which they occur is 50–55 years, but they may occur at any age.

Anti-Müllerian hormone (AMH), also known as Müllerian-inhibiting hormone (MIH), is a glycoprotein hormone structurally related to inhibin and activin from the transforming growth factor beta superfamily, whose key roles are in growth differentiation and folliculogenesis. In humans, it is encoded by the AMH gene, on chromosome 19p13.3, while its receptor is encoded by the AMHR2 gene on chromosome 12.

FOXP3, also known as scurfin, is a protein involved in immune system responses. A member of the FOX protein family, FOXP3 appears to function as a master regulator of the regulatory pathway in the development and function of regulatory T cells. Regulatory T cells generally turn the immune response down. In cancer, an excess of regulatory T cell activity can prevent the immune system from destroying cancer cells. In autoimmune disease, a deficiency of regulatory T cell activity can allow other autoimmune cells to attack the body's own tissues.

Growth/differentiation factor 9 is a protein that in humans is encoded by the GDF9 gene.
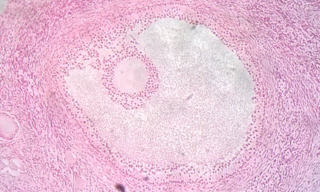
Follicular atresia refers to the process in which a follicle fails to develop, thus preventing it from ovulating and releasing an egg. It is a normal, naturally occurring progression that occurs as mammalian ovaries age. Approximately 1% of mammalian follicles in ovaries undergo ovulation and the remaining 99% of follicles go through follicular atresia as they cycle through the growth phases. In summary, follicular atresia is a process that leads to the follicular loss and loss of oocytes, and any disturbance or loss of functionality of this process can lead to many other conditions.
Primary ovarian insufficiency (POI), also called premature ovarian insufficiency, premature menopause, and premature ovarian failure, is the partial or total loss of reproductive and hormonal function of the ovaries before age 40 because of follicular dysfunction or early loss of eggs. POI can be seen as part of a continuum of changes leading to menopause that differ from age-appropriate menopause in the age of onset, degree of symptoms, and sporadic return to normal ovarian function. POI affects approximately 1 in 10,000 women under age 20, 1 in 1,000 women under age 30, and 1 in 100 of those under age 40. A medical triad for the diagnosis is amenorrhea, hypergonadotropism, and hypoestrogenism.

Bone morphogenetic protein 15 (BMP-15) is a protein that in humans is encoded by the BMP15 gene. It is involved in folliculogenesis, the process in which primordial follicles develop into pre-ovulatory follicles.
XX gonadal dysgenesis is a type of female hypogonadism in which the ovaries do not function to induce puberty in an otherwise normal girl whose karyotype is found to be 46,XX. With nonfunctional streak ovaries, she is low in estrogen levels (hypoestrogenic) and has high levels of FSH and LH. Estrogen and progesterone therapy is usually then commenced. Some cases are considered a severe version of premature ovarian failure where the ovaries fail before puberty.
Forkhead box protein C2 (FOXC2) also known as forkhead-related protein FKHL14 (FKHL14), transcription factor FKH-14, or mesenchyme fork head protein 1 (MFH1) is a protein that in humans is encoded by the FOXC2 gene. FOXC2 is a member of the fork head box (FOX) family of transcription factors.

Blepharophimosis is a congenital anomaly in which the eyelids are underdeveloped such that they cannot open as far as usual and permanently cover part of the eyes. Both the vertical and horizontal palpebral fissures are shortened; the eyes also appear spaced more widely apart as a result, known as telecanthus.

Forkhead box C1, also known as FOXC1, is a protein which in humans is encoded by the FOXC1 gene.
T-box transcription factor TBX5, is a protein that in humans is encoded by the TBX5 gene. Abnormalities in the TBX5 gene can result in altered limb development, Holt-Oram syndrome, Tetra-amelia syndrome, and cardiac and skeletal problems.

Forkhead box protein E1 is a protein that in humans is encoded by the FOXE1 gene.

28S ribosomal protein S22, mitochondrial is a protein that in humans is encoded by the MRPS22 gene.

Transcription factor SOX-14 is a protein that in humans is encoded by the SOX14 gene.
Forkhead box D3 also known as FOXD3 is a forkhead protein that in humans is encoded by the FOXD3 gene.

Folliculogenesis-specific basic helix-loop-helix, also known as factor in the germline alpha (FIGalpha) or transcription factor FIGa, is a protein that in humans is encoded by the FIGLA gene. The FIGLA gene is a germ cell-specific transcription factor preferentially expressed in oocytes that can be found on human chromosome 2p13.3.

Forkhead box protein E3 (FOXE3) also known as forkhead-related transcription factor 8 (FREAC-8) is a protein that in humans is encoded by the FOXE3 gene located on the short arm of chromosome 1.

Blepharophimosis, ptosis, epicanthus inversus syndrome (BPES) is a rare medical anomaly characterized by the conditions it is named after: blepharophimosis, ptosis and epicanthus inversus. There are two types; type 1 is distinguished from type 2 by including the symptom of premature ovarian insufficiency (POI) in females, which causes menopausal symptoms and infertility in patients as young as 15 years old.
Ovarian follicle activation can be defined as primordial follicles in the ovary moving from a quiescent (inactive) to a growing phase. The primordial follicle in the ovary is what makes up the “pool” of follicles that will be induced to enter growth and developmental changes that change them into pre-ovulatory follicles, ready to be released during ovulation. The process of development from a primordial follicle to a pre-ovulatory follicle is called folliculogenesis.