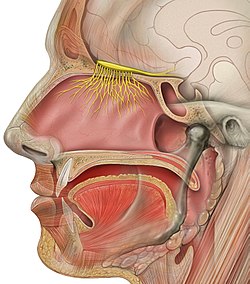
The vomeronasal organ (VNO), or Jacobson's organ, is the paired auxiliary olfactory (smell) sense organ located in the soft tissue of the nasal septum, in the nasal cavity just above the roof of the mouth in various tetrapods. The name is derived from the fact that it lies adjacent to the unpaired vomer bone in the nasal septum. It is present and functional in all snakes and lizards, and in many mammals, including cats, dogs, cattle, pigs, and some primates. Some humans may have physical remnants of a VNO, but it is vestigial and non-functional.

The nasal cavity is a large, air-filled space above and behind the nose in the middle of the face. The nasal septum divides the cavity into two cavities, also known as fossae. Each cavity is the continuation of one of the two nostrils. The nasal cavity is the uppermost part of the respiratory system and provides the nasal passage for inhaled air from the nostrils to the nasopharynx and rest of the respiratory tract.

The olfactory bulb is a neural structure of the vertebrate forebrain involved in olfaction, the sense of smell. It sends olfactory information to be further processed in the amygdala, the orbitofrontal cortex (OFC) and the hippocampus where it plays a role in emotion, memory and learning. The bulb is divided into two distinct structures: the main olfactory bulb and the accessory olfactory bulb. The main olfactory bulb connects to the amygdala via the piriform cortex of the primary olfactory cortex and directly projects from the main olfactory bulb to specific amygdala areas. The accessory olfactory bulb resides on the dorsal-posterior region of the main olfactory bulb and forms a parallel pathway. Destruction of the olfactory bulb results in ipsilateral anosmia, while irritative lesions of the uncus can result in olfactory and gustatory hallucinations.
A chemoreceptor, also known as chemosensor, is a specialized sensory receptor which transduces a chemical substance to generate a biological signal. This signal may be in the form of an action potential, if the chemoreceptor is a neuron, or in the form of a neurotransmitter that can activate a nerve fiber if the chemoreceptor is a specialized cell, such as taste receptors, or an internal peripheral chemoreceptor, such as the carotid bodies. In physiology, a chemoreceptor detects changes in the normal environment, such as an increase in blood levels of carbon dioxide (hypercapnia) or a decrease in blood levels of oxygen (hypoxia), and transmits that information to the central nervous system which engages body responses to restore homeostasis.

In physiology, a stimulus is a detectable change in the physical or chemical structure of an organism's internal or external environment. The ability of an organism or organ to detect external stimuli, so that an appropriate reaction can be made, is called sensitivity (excitability). Sensory receptors can receive information from outside the body, as in touch receptors found in the skin or light receptors in the eye, as well as from inside the body, as in chemoreceptors and mechanoreceptors. When a stimulus is detected by a sensory receptor, it can elicit a reflex via stimulus transduction. An internal stimulus is often the first component of a homeostatic control system. External stimuli are capable of producing systemic responses throughout the body, as in the fight-or-flight response. In order for a stimulus to be detected with high probability, its level of strength must exceed the absolute threshold; if a signal does reach threshold, the information is transmitted to the central nervous system (CNS), where it is integrated and a decision on how to react is made. Although stimuli commonly cause the body to respond, it is the CNS that finally determines whether a signal causes a reaction or not.

The olfactory system or sense of smell is the sensory system used for smelling (olfaction). Olfaction is one of the special senses, that have directly associated specific organs. Most mammals and reptiles have a main olfactory system and an accessory olfactory system. The main olfactory system detects airborne substances, while the accessory system senses fluid-phase stimuli.

An olfactory receptor neuron (ORN), also called an olfactory sensory neuron (OSN), is a sensory neuron within the olfactory system.

The olfactory epithelium is a specialized epithelial tissue inside the nasal cavity that is involved in smell. In humans, it measures 5 cm2 (0.78 sq in) and lies on the roof of the nasal cavity about 7 cm (2.8 in) above and behind the nostrils. The olfactory epithelium is the part of the olfactory system directly responsible for detecting odors.
The olfactory mucosa is the neuroepithelialial mucosa lining the roof and upper parts of the septum and lateral wall of the nasal cavity which contains bipolar neurons of the primary receptor neurons of the olfactory pathway, as well as supporting cells. The neurons' dendrites project towards the nasal cavity while their axons ascend through the cribriform plate as the olfactory nerves.

The glomerulus is a spherical structure located in the olfactory bulb of the brain where synapses form between the terminals of the olfactory nerve and the dendrites of mitral, periglomerular and tufted cells. Each glomerulus is surrounded by a heterogeneous population of juxtaglomerular neurons and glial cells.

Sensory neurons, also known as afferent neurons, are neurons in the nervous system, that convert a specific type of stimulus, via their receptors, into action potentials or graded receptor potentials. This process is called sensory transduction. The cell bodies of the sensory neurons are located in the dorsal ganglia of the spinal cord.
In medicine and anatomy, the special senses are the senses that have specialized organs devoted to them:

Mitral cells are neurons that are part of the olfactory system. They are located in the olfactory bulb in the mammalian central nervous system. They receive information from the axons of olfactory receptor neurons, forming synapses in neuropils called glomeruli. Axons of the mitral cells transfer information to a number of areas in the brain, including the piriform cortex, entorhinal cortex, and amygdala. Mitral cells receive excitatory input from olfactory sensory neurons and external tufted cells on their primary dendrites, whereas inhibitory input arises either from granule cells onto their lateral dendrites and soma or from periglomerular cells onto their dendritic tuft. Mitral cells together with tufted cells form an obligatory relay for all olfactory information entering from the olfactory nerve. Mitral cell output is not a passive reflection of their input from the olfactory nerve. In mice, each mitral cell sends a single primary dendrite into a glomerulus receiving input from a population of olfactory sensory neurons expressing identical olfactory receptor proteins, yet the odor responsiveness of the 20-40 mitral cells connected to a single glomerulus is not identical to the tuning curve of the input cells, and also differs between sister mitral cells. Odorant response properties of individual neurons in an olfactory glomerular module. The exact type of processing that mitral cells perform with their inputs is still a matter of controversy. One prominent hypothesis is that mitral cells encode the strength of an olfactory input into their firing phases relative to the sniff cycle. A second hypothesis is that the olfactory bulb network acts as a dynamical system that decorrelates to differentiate between representations of highly similar odorants over time. Support for the second hypothesis comes primarily from research in zebrafish.
A topographic map is the ordered projection of a sensory surface, like the retina or the skin, or an effector system, like the musculature, to one or more structures of the central nervous system. Topographic maps can be found in all sensory systems and in many motor systems.

The anterior olfactory nucleus is a portion of the forebrain of vertebrates.
Olfactory fatigue, also known as odor fatigue, olfactory adaptation, and noseblindness, is the temporary, normal inability to distinguish a particular odor after a prolonged exposure to that airborne compound. For example, when entering a restaurant initially the odor of food is often perceived as being very strong, but after time the awareness of the odor normally fades to the point where the smell is not perceptible or is much weaker. After leaving the area of high odor, the sensitivity is restored with time. Anosmia is the permanent loss of the sense of smell, and is different from olfactory fatigue.
Dysosmia is a disorder described as any qualitative alteration or distortion of the perception of smell. Qualitative alterations differ from quantitative alterations, which include anosmia and hyposmia. Dysosmia can be classified as either parosmia or phantosmia. Parosmia is a distortion in the perception of an odorant. Odorants smell different from what one remembers. Phantosmia is the perception of an odor when no odorant is present. The cause of dysosmia still remains a theory. It is typically considered a neurological disorder and clinical associations with the disorder have been made. Most cases are described as idiopathic and the main antecedents related to parosmia are URTIs, head trauma, and nasal and paranasal sinus disease. Dysosmia tends to go away on its own but there are options for treatment for patients that want immediate relief.

The sense of smell, or olfaction, is the special sense through which smells are perceived. The sense of smell has many functions, including detecting desirable foods, hazards, and pheromones, and plays a role in taste.

Insect olfaction refers to the function of chemical receptors that enable insects to detect and identify volatile compounds for foraging, predator avoidance, finding mating partners and locating oviposition habitats. Thus, it is the most important sensation for insects. Most important insect behaviors must be timed perfectly which is dependent on what they smell and when they smell it. For example, olfaction is essential for locating host plants and hunting prey in many species of insects, such as the moth Deilephila elpenor and the wasp Polybia sericea, respectively.
Retronasal smell, retronasal olfaction, is the ability to perceive flavor dimensions of foods and drinks. Retronasal smell is a sensory modality that produces flavor. It is best described as a combination of traditional smell and taste modalities. Retronasal smell creates flavor from smell molecules in foods or drinks shunting up through the nasal passages as one is chewing. When people use the term "smell", they are usually referring to "orthonasal smell", or the perception of smell molecules that enter directly through the nose and up the nasal passages. Retronasal smell is critical for experiencing the flavor of foods and drinks. Flavor should be contrasted with taste, which refers to five specific dimensions: (1) sweet, (2) salty, (3) bitter, (4) sour, and (5) umami. Perceiving anything beyond these five dimensions, such as distinguishing the flavor of an apple from a pear for example, requires the sense of retronasal smell.