
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings sandwiching a central iron atom. It is an orange solid with a camphor-like odor that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2. Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and Fc+ respectively.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

n-Butyllithium C4H9Li (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly employed as a strong base (superbase) in the synthesis of organic compounds as in the pharmaceutical industry.

tert-Butyllithium is a chemical compound with the formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon molecules, including benzene. tert-Butyllithium is available commercially as solutions in hydrocarbons (such as pentane); it is not usually prepared in the laboratory.

Directed ortho metalation (DoM) is an adaptation of electrophilic aromatic substitution in which electrophiles attach themselves exclusively to the ortho- position of a direct metalation group or DMG through the intermediary of an aryllithium compound. The DMG interacts with lithium through a hetero atom. Examples of DMG's are the methoxy group, a tertiary amine group and an amide group.The compound can be produced by directed lithiation of anisole.
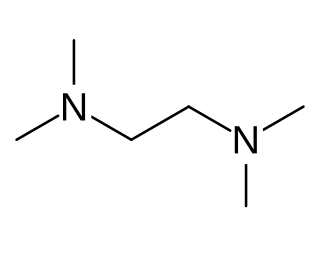
Tetramethylethylenediamine (TMEDA or TEMED) is a chemical compound with the formula (CH3)2NCH2CH2N(CH3)2. This species is derived from ethylenediamine by replacement of the four amine hydrogens with four methyl groups. It is a colorless liquid, although old samples often appear yellow. Its odor is similar to that of rotting fish.

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives. Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.

1,1′-Bis(diphenylphosphino)ferrocene, commonly abbreviated dppf, is an organophosphorus compound commonly used as a ligand in homogeneous catalysis. It contains a ferrocene moiety in its backbone, and is related to other bridged diphosphines such as 1,2-bis(diphenylphosphino)ethane (dppe).

Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Nickel(II) bis(acetylacetonate) is a coordination complex with the formula [Ni(acac)2]3, where acac is the anion C5H7O−2 derived from deprotonation of acetylacetone. It is a dark green paramagnetic solid that is soluble in organic solvents such as toluene. It reacts with water to give the blue-green diaquo complex Ni(acac)2(H2O)2.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).

Transition metal alkyl complexes are coordination complexes that contain a bond between a transition metal and an alkyl ligand. Such complexes are not only pervasive but are of practical and theoretical interest.
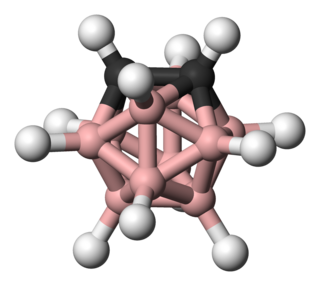
ortho-Carborane is the organoboron compound with the formula C2B10H12. The prefix ortho is derived from ortho. It is the most prominent carborane. This derivative has been considered for a wide range of applications from heat-resistant polymers to medical applications. It is a colorless solid that melts, without decomposition, at 320 °C

Lithium cyclopentadienide is an organolithium compound with the formula C5H5Li. The compound is often abbreviated as LiCp, where Cp− is the cyclopentadienide anion. Lithium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.

Lithium naphthalene is an organic salt with the chemical formula Li+[C10H8]−. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. Lithium naphthalene crystallizes with ligands bound to Li+. The anion is a well-known example of an organic radical.

(Trimethylsilyl)methyllithium is classified both as an organolithium compound and an organosilicon compound. It has the empirical formula LiCH2Si(CH3)3, often abbreviated LiCH2TMS. It crystallizes as the hexagonal prismatic hexamer [LiCH2TMS]6, akin to some polymorphs of methyllithium. Many adducts have been characterized including the diethyl ether complexed cubane [Li4(μ3-CH2TMS)4(Et2O)2] and [Li2(μ-CH2TMS)2(TMEDA)2].

Tetramethylethylenediamine(dimethyl)nickel(II) is the organonickel complex with the formula (Me2NCH2CH2NMe2)NiMe2 (Me = CH3). This yellow-brown, air-sensitive compound is popular precursor to diverse organonickel complexes. It is prepared from the tmeda adduct of nickel(II) acetylacetonate by reaction with methyl lithium.

Tetrakis(1-norbornyl)cobalt(IV) is an air-sensitive organometallic compound of cobalt. It was first synthesized by Barton K. Bower and Howard G. Tennent in 1972 and is one of few compounds in which cobalt has a formal oxidation state of +4.



















