| |||
 | |||
 | |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name | |||
| Other names Octasulfur | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| 2973 | |||
| MeSH | Cyclooctasulfur | ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
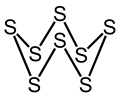
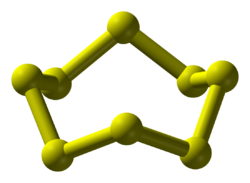
| S8 | |||
| Molar mass | 256.48 g·mol−1 | ||
| Appearance | Vivid, yellow, translucent crystals | ||
| Density | 2.07 g/cm3 | ||
| Melting point | 119 °C; 246 °F; 392 K | ||
| Boiling point | 444.6 °C; 832.4 °F; 717.8 K | ||
| log P | 6.117 | ||
| Thermochemistry | |||
Std molar entropy (S⦵298) | 32 J·mol−1·K−1 [3] | ||
Std enthalpy of formation (ΔfH⦵298) | 0 kJ/mol [3] | ||
| Related compounds | |||
Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Octasulfur is an inorganic substance with the chemical formula S8. It is an odourless and tasteless yellow solid, and is a major industrial chemical. It is the most common allotrope of sulfur and occurs widely in nature. [4]