In chemistry, an acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory.

In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and more frequently in the past) been applied to all compounds containing covalently bound H atoms. In this broad and potentially archaic sense, water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. In covalent compounds, it implies hydrogen is attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure R−CH2−C6H5. Benzyl features a benzene ring attached to a methylene group.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .

Hydrogen iodide (HI) is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent.

n-Butyllithium C4H9Li (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly employed as a strong base (superbase) in the synthesis of organic compounds as in the pharmaceutical industry.
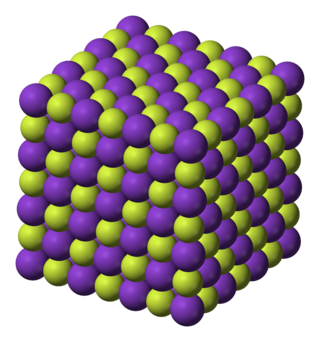
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide salt and occurs naturally as the rare mineral carobbiite. Solutions of KF will etch glass due to the formation of soluble fluorosilicates, although HF is more effective.
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The chemical energy released in the formation of non-covalent interactions is typically on the order of 1–5 kcal/mol. Non-covalent interactions can be classified into different categories, such as electrostatic, π-effects, van der Waals forces, and hydrophobic effects.
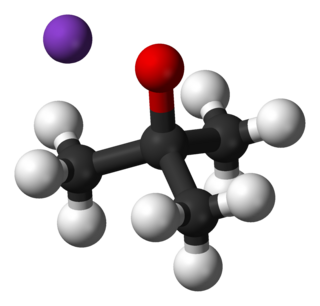
Potassium tert-butoxide (or potassium t-butoxide) is a chemical compound with the formula [(CH3)3COK]n (abbr. KOtBu). This colourless solid is a strong base (pKa of conjugate acid around 17), which is useful in organic synthesis. The compound is often depicted as a salt, and it often behaves as such, but its ionization depends on the solvent.

Tetrafluoroborate is the anion BF−
4. This tetrahedral species is isoelectronic with tetrafluoroberyllate (BeF2−
4), tetrafluoromethane (CF4), and tetrafluoroammonium (NF+
4) and is valence isoelectronic with many stable and important species including the perchlorate anion, ClO−
4, which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF3, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid.

Grignard reagents or Grignard compounds are chemical compounds with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.

Indium(III) chloride is the chemical compound with the formula InCl3 which forms a tetrahydrate. This salt is a white, flaky solid with applications in organic synthesis as a Lewis acid. It is also the most available soluble derivative of indium. This is one of three known indium chlorides.

Methyllithium is the simplest organolithium reagent, with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive towards water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers.
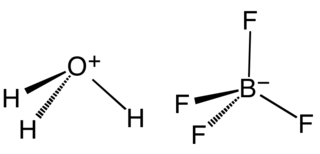
Fluoroboric acid or tetrafluoroboric acid is an inorganic compound with the simplified chemical formula H+[BF4]−. Solvent-free tetrafluoroboric acid has not been reported. The term "fluoroboric acid" usually refers to a range of compounds including hydronium tetrafluoroborate, which are available as solutions. The ethyl ether solvate is also commercially available, where the fluoroboric acid can be represented by the formula [H( 2O)n]+[BF4]−, where n is 2.

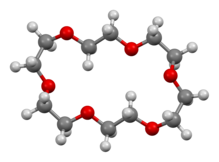
12-Crown-4, also called 1,4,7,10-tetraoxacyclododecane and lithium ionophore V, is a crown ether with the formula C8H16O4. It is a cyclic tetramer of ethylene oxide which is specific for the lithium cation.

15-Crown-5 is a crown ether with the formula (C2H4O)5. It is a cyclic pentamer of ethylene oxide that forms complex with various cations, including sodium (Na+) and potassium (K+); however, it is complementary to Na+ and thus has a higher selectivity for Na+ ions.

Silver trifluoromethanesulfonate, or silver triflate is the triflate (CF3SO3−) salt of Ag+. It is a white or colorless solid that is soluble in water and some organic solvents including, benzene. It is a reagent used in the synthesis of organic and inorganic triflates.
An arsinide, arsanide, dihydridoarsenate(1−) or arsanyl compound is a chemical derivative of arsine, where one hydrogen atom is replaced with a metal or cation. The arsinide ion has formula AsH−2. It can be considered as a ligand with name arsenido or arsanido. Few chemists study arsanyl compounds, as they are both toxic and unstable. The IUPAC names are arsanide and dihydridoarsenate(1−). For the ligand the name is arsanido. The neutral −AsH2 group is termed arsanyl.

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.