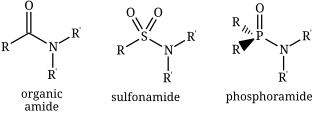
An amide, also known as an acid amide, is a compound with the functional group RnE(O)xNR′2. Most common are carboxamides, but many other important types of amides are known, including phosphoramides and sulfonamides. The term amide refers both to classes of compounds and to the functional group within those compounds.

In chemistry, an ester − as opposed to an ether − is a chemical compound derived from an acid in which at least one –OH (hydroxyl) group is replaced by an –O–alkyl (alkoxy) group. Usually, esters are derived from a carboxylic acid and an alcohol. Glycerides, which are fatty acid esters of glycerol, are important esters in biology, being one of the main classes of lipids, and making up the bulk of animal fats and vegetable oils. Esters with low molecular weight are commonly used as fragrances and found in essential oils and pheromones. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties, while polyesters are important plastics, with monomers linked by ester moieties. Esters usually have a sweet smell and are considered high-quality solvents for a broad array of plastics, plasticizers, resins, and lacquers. They are also one of the largest classes of synthetic lubricants on the commercial market.

A ketene is an organic compound of the form R′R″C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refers to the specific compound ethenone H
2C=C=O, the simplest ketene.
Viscose is a term often used to represent the viscose fiber that is made from natural sources such as wood and agricultural products that are regenerated as cellulose fiber. The molecular structure of natural cellulose is preserved in the process. The many types and grades of viscose fibers can imitate the feel and texture of natural fibers such as silk, wool, cotton, and linen. The types that resemble silk are often called artificial silk.</ref> The fibre is used to make textiles for clothing and other purposes.

In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group -COCl. Their formula is usually written RCOCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant toward hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides.

Acetyl chloride (CH3COCl) is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acyl halides. It is a colorless, corrosive, volatile liquid.
The Leimgruber–Batcho indole synthesis is a series of organic reactions that produce indoles from o-nitrotoluenes 1. The first step is the formation of an enamine 2 using N,N-dimethylformamide dimethyl acetal and pyrrolidine. The desired indole 3 is then formed in a second step by reductive cyclisation.
The Madelung synthesis is a chemical reaction that produces indoles by the intramolecular cyclization of N-phenylamides using strong base at high temperature.The Madelung synthesis was reported in 1912 by Walter Madelung, when he observed that 2-phenylindole was synthesized using N-benzoyl-o-toluidine and two equivalents of sodium ethoxide in a heated, airless, reaction. Common reaction conditions include use of sodium or potassium alkoxide as base in hexane or tetrahydrofuran solvents, at temperatures ranging between 200–400 °C. A hydrolysis step is also required in the synthesis. The Madelung synthesis is important because it is one of few known reactions that produce indoles from a base-catalyzed thermal cyclization of N-acyl-o-toluidines. The overall reaction for the Madelung synthesis follows.
Aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid functional group in an electrophilic aromatic substitution. Aryl sulfonic acids are used as detergents, dye, and drugs.

The Curtius rearrangement, first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a variety of nucleophiles such as water, alcohols and amines, to yield a primary amine, carbamate or urea derivative respectively. Several reviews have been published.

Hydrazides in organic chemistry are a class of organic compounds sharing a common functional group characterized by hydrazine core in which at least one of the hydrogen atoms is replaced by a substituent that is an acyl group. The general structure for a hydrazide is E(=O)-NR-NR2, where the R's are frequently hydrogens. Hydrazides can be further classified by atom attached to the oxygen: carbohydrazides (R-C(=O)-NH-NH2), sulfonohydrazides (R-S(=O)2-NH-NH2) and phosphonic dihydrazides (R-P(=O)(-NH-NH2)2. The related hydrazines do not carry an acyl group. Some important members of this class are sulfonylhydrazides such as p-toluenesulfonylhydrazide which are useful reagents in organic chemistry such as in the Shapiro reaction and the Eschenmoser–Tanabe fragmentation. This reagent can be prepared by reaction of tosyl chloride with hydrazine.

Crotonic acid ((2E)-but-2-enoic acid) or is a short-chain unsaturated carboxylic acid, described by the formula CH3CH=CHCO2H. It is called crotonic acid because it was erroneously thought to be a saponification product of croton oil. It crystallizes as colorless needles from hot water. The cis-isomer of crotonic acid is called isocrotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to butyric acid.

Pyridazine is a heterocyclic organic compound with the molecular formula (CH)4N2. It contains a six-membered ring with two adjacent nitrogen atoms, and is aromatic. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other (CH)4N2 rings, pyrimidine and pyrazine.
Aminolysis (/am·i·nol·y·sis/ amino meaning "contains NH2 group", and lysis meaning "to unbind") is any chemical compound reacts with a molecule of ammonia or an amine and causes a molecule to split into two parts, containing the addition of (or substitution by) an amino group —NH—. The subset of aminolysis reactions involving ammonia is known as ammonolysis.

Hydrazines (R2N-NR2) are a class of chemical compounds which have two nitrogen atoms linked via a covalent bond and which carry from one up to four alkyl or aryl substituents. Hydrazines can be considered as derivatives of the inorganic hydrazine (H2N-NH2), in which one or more hydrogen atoms have been replaced by hydrocarbon groups.

Sulfanilic acid is an off-white crystalline solid which finds application in quantitative analysis of nitrate and nitrite ions. The solid acid exists as a zwitterion, and has an unusually high melting point.
The alkylbenzenes are derivatives of benzene, in which one or more hydrogen atoms are replaced by alkyl groups of different sizes. They are a subset of the aromatic hydrocarbons. The simplest member is toluene, in which a hydrogen atom of the benzene was replaced by a methyl group.
























