
Stanley Ben Prusiner is an American neurologist and biochemist. He is the director of the Institute for Neurodegenerative Diseases at University of California, San Francisco (UCSF). Prusiner discovered prions, a class of infectious self-reproducing pathogens primarily or solely composed of protein, considered by many as a heretical idea when first proposed. He received the Albert Lasker Award for Basic Medical Research in 1994 and the Nobel Prize in Physiology or Medicine in 1997 for prion research developed by him and his team of experts beginning in the early 1970s.

In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis.

Avram Hershko is a Hungarian-Israeli biochemist who received the Nobel Prize in Chemistry in 2004.

Joseph Leonard Goldstein ForMemRS is an American biochemist. He received the Nobel Prize in Physiology or Medicine in 1985, along with fellow University of Texas Southwestern researcher, Michael Brown, for their studies regarding cholesterol. They discovered that human cells have low-density lipoprotein (LDL) receptors that remove cholesterol from the blood and that when LDL receptors are not present in sufficient numbers, individuals develop hypercholesterolemia and become at risk for cholesterol related diseases, notably coronary heart disease. Their studies led to the development of statin drugs.

GroEL is a protein which belongs to the chaperonin family of molecular chaperones, and is found in many bacteria. It is required for the proper folding of many proteins. To function properly, GroEL requires the lid-like cochaperonin protein complex GroES. In eukaryotes the organellar proteins Hsp60 and Hsp10 are structurally and functionally nearly identical to GroEL and GroES, respectively, due to their endosymbiotic origin.
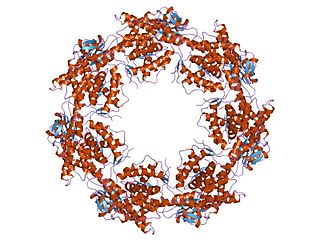
HSP60, also known as chaperonins (Cpn), is a family of heat shock proteins originally sorted by their 60kDa molecular mass. They prevent misfolding of proteins during stressful situations such as high heat, by assisting protein folding. HSP60 belong to a large class of molecules that assist protein folding, called molecular chaperones.

Robert G. Roeder is an American biochemist. He is known as a pioneer scientist in eukaryotic transcription. He discovered three distinct nuclear RNA polymerases in 1969 and characterized many proteins involved in the regulation of transcription, including basic transcription factors and the first mammalian gene-specific activator over five decades of research. He is the recipient of the Gairdner Foundation International Award in 2000, the Albert Lasker Award for Basic Medical Research in 2003, and the Kyoto Prize in 2021. He currently serves as Arnold and Mabel Beckman Professor and Head of the Laboratory of Biochemical and Molecular Biology at The Rockefeller University.

James Edward Rothman is an American biochemist. He is the Fergus F. Wallace Professor of Biomedical Sciences at Yale University, the Chairman of the Department of Cell Biology at Yale School of Medicine, and the Director of the Nanobiology Institute at the Yale West Campus. Rothman also concurrently serves as adjunct professor of physiology and cellular biophysics at Columbia University and a research professor at the UCL Queen Square Institute of Neurology, University College London.
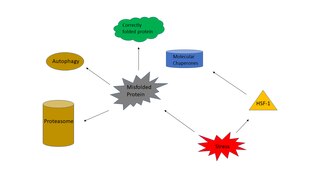
The heat shock response (HSR) is a cell stress response that increases the number of molecular chaperones to combat the negative effects on proteins caused by stressors such as increased temperatures, oxidative stress, and heavy metals. In a normal cell, proteostasis must be maintained because proteins are the main functional units of the cell. Many proteins take on a defined configuration in a process known as protein folding in order to perform their biological functions. If these structures are altered, critical processes could be affected, leading to cell damage or death. The heat shock response can be employed under stress to induce the expression of heat shock proteins (HSP), many of which are molecular chaperones, that help prevent or reverse protein misfolding and provide an environment for proper folding.

Oliver Smithies was a British-American geneticist and physical biochemist. He is known for introducing starch as a medium for gel electrophoresis in 1955, and for the discovery, simultaneously with Mario Capecchi and Martin Evans, of the technique of homologous recombination of transgenic DNA with genomic DNA, a much more reliable method of altering animal genomes than previously used, and the technique behind gene targeting and knockout mice. He received the Nobel Prize in Physiology or Medicine in 2007 for his genetics work.
Bertil Hille is an Emeritus Professor, and the Wayne E. Crill Endowed Professor in the Department of Physiology and Biophysics at the University of Washington. He is particularly well known for his pioneering research on cell signalling by ion channels. His book Ion Channels of Excitable Membranes has been the standard work on the subject, appearing in multiple editions since its first publication in 1984.

Michael Grunstein is a Distinguished Professor Emeritus of Biological Chemistry at the David Geffen School of Medicine at UCLA.

Roger David Kornberg is an American biochemist and professor of structural biology at Stanford University School of Medicine. Kornberg was awarded the Nobel Prize in Chemistry in 2006 for his studies of the process by which genetic information from DNA is copied to RNA, "the molecular basis of eukaryotic transcription."

Joan Elaine Argetsinger Steitz is Sterling Professor of Molecular Biophysics and Biochemistry at Yale University and Investigator at the Howard Hughes Medical Institute. She is known for her discoveries involving RNA, including ground-breaking insights into how ribosomes interact with messenger RNA by complementary base pairing and that introns are spliced by small nuclear ribonucleic proteins (snRNPs), which occur in eukaryotes. In September 2018, Steitz won the Lasker-Koshland Award for Special Achievement in Medical Science. The Lasker award is often referred to as the 'American Nobel' because 87 of the former recipients have gone on to win Nobel prizes.

Carolyn Widney Greider is an American molecular biologist and Nobel laureate. She joined the University of California, Santa Cruz as a Distinguished Professor in the department of molecular, cell, and developmental biology in October 2020.
Franz-Ulrich Hartl is a German biochemist and the current Executive Director of the Max Planck Institute of Biochemistry. He is known for his pioneering work in chaperone-mediated protein folding.
The Wiley Prize in Biomedical Sciences is intended to recognize breakthrough research in pure or applied life science research that is distinguished by its excellence, originality and impact on our understanding of biological systems and processes. The award may recognize a specific contribution or series of contributions that demonstrate the nominee's significant leadership in the development of research concepts or their clinical application. Particular emphasis will be placed on research that champions novel approaches and challenges accepted thinking in the biomedical sciences.

Sir Peter John Ratcliffe, FRS, FMedSci is a British physician-scientist who is trained as a nephrologist. He was a practising clinician at the John Radcliffe Hospital, Oxford and Nuffield Professor of Clinical Medicine and head of the Nuffield Department of Clinical Medicine at the University of Oxford from 2004 to 2016. He has been a Fellow of Magdalen College, Oxford since 2004. In 2016 he became Clinical Research Director at the Francis Crick Institute, retaining a position at Oxford as a member of the Ludwig Institute of Cancer Research and director of the Target Discovery Institute, University of Oxford.

Gregg Leonard Semenza is a pediatrician and Professor of Genetic Medicine at the Johns Hopkins School of Medicine. He serves as the director of the vascular program at the Institute for Cell Engineering. He is a 2016 recipient of the Albert Lasker Award for Basic Medical Research. He is known for his discovery of HIF-1, which allows cancer cells to adapt to oxygen-poor environments. He shared the 2019 Nobel Prize in Physiology or Medicine for "discoveries of how cells sense and adapt to oxygen availability" with William Kaelin Jr. and Peter J. Ratcliffe. Semenza has had ten research papers retracted due to falsified data.

William G. Kaelin Jr. is an American Nobel laureate physician-scientist. He is a professor of medicine at Harvard University and the Dana–Farber Cancer Institute. His laboratory studies tumor suppressor proteins. In 2016, Kaelin received the Albert Lasker Award for Basic Medical Research and the AACR Princess Takamatsu Award. He also won the Nobel Prize in Physiology or Medicine in 2019 along with Peter J. Ratcliffe and Gregg L. Semenza.

















