
Aflatoxins are various poisonous carcinogens and mutagens that are produced by certain molds, particularly Aspergillus species. The fungi grow in soil, decaying vegetation and various staple foodstuffs and commodities such as hay, sweetcorn, wheat, millet, sorghum, cassava, rice, chili peppers, cottonseed, peanuts, tree nuts, sesame seeds, sunflower seeds, and various spices. In short, the relevant fungi grow on almost any crop or food. When such contaminated food is processed or consumed, the aflatoxins enter the general food supply. They have been found in both pet and human foods, as well as in feedstocks for agricultural animals. Animals fed contaminated food can pass aflatoxin transformation products into eggs, milk products, and meat. For example, contaminated poultry feed is the suspected source of aflatoxin-contaminated chicken meat and eggs in Pakistan.

Aspergillus niger is a mold classified within the Nigri section of the Aspergillus genus. The Aspergillus genus consists of common molds found throughout the environment within soil and water, on vegetation, in fecal matter, on decomposing matter, and suspended in the air. Species within this genus often grow quickly and can sporulate within a few days of germination. A combination of characteristics unique to A. niger makes the microbe invaluable to the production of many acids, proteins and bioactive compounds. Characteristics including extensive metabolic diversity, high production yield, secretion capability, and the ability to conduct post-translational modifications are responsible for A. niger's robust production of secondary metabolites. A. niger's capability to withstand extremely acidic conditions makes it especially important to the industrial production of citric acid.
A mycotoxin is a toxic secondary metabolite produced by fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops.

Aspergillus flavus is a saprotrophic and pathogenic fungus with a cosmopolitan distribution. It is best known for its colonization of cereal grains, legumes, and tree nuts. Postharvest rot typically develops during harvest, storage, and/or transit. Its specific name flavus derives from the Latin meaning yellow, a reference to the frequently observed colour of the spores. A. flavus infections can occur while hosts are still in the field (preharvest), but often show no symptoms (dormancy) until postharvest storage or transport. In addition to causing preharvest and postharvest infections, many strains produce significant quantities of toxic compounds known as mycotoxins, which, when consumed, are toxic to mammals. A. flavus is also an opportunistic human and animal pathogen, causing aspergillosis in immunocompromised individuals.

Aspergillus is a genus consisting of several hundred mould species found in various climates worldwide.

Aspergillus oryzae, also known as kōji mold, is a filamentous fungus used in East Asia to saccharify rice, sweet potato, and barley in the making of alcoholic beverages such as sake and shōchū, and also to ferment soybeans for making soy sauce and miso. However, in the production of fermented foods of soybeans such as soy sauce and miso, Aspergillus sojae is sometimes used instead of A. oryzae. Incidentally, in China and Korea, the fungi used for fermented foods for a long time in the production of traditional alcoholic beverages were not A. oryzae but fungi belonging to Rhizopus and Mucor. A. oryzae is also used for the production of rice vinegars. Barley kōji (麦麹) or rice kōji (米麹) are made by fermenting the grains with A. oryzae hyphae.

Rhizopus oligosporus is a fungus of the family Mucoraceae and is a widely used starter culture for the production of tempeh at home and industrially. As the mold grows it produces fluffy, white mycelia, binding the beans together to create an edible "cake" of partly catabolized soybeans. The domestication of the microbe is thought to have occurred in Indonesia several centuries ago.
Mycotoxicology is the branch of mycology that focuses on analyzing and studying the toxins produced by fungi, known as mycotoxins. In the food industry it is important to adopt measures that keep mycotoxin levels as low as practicable, especially those that are heat-stable. These chemical compounds are the result of secondary metabolism initiated in response to specific developmental or environmental signals. This includes biological stress from the environment, such as lower nutrients or competition for those available. Under this secondary path the fungus produces a wide array of compounds in order to gain some level of advantage, such as incrementing the efficiency of metabolic processes to gain more energy from less food, or attacking other microorganisms and being able to use their remains as a food source.
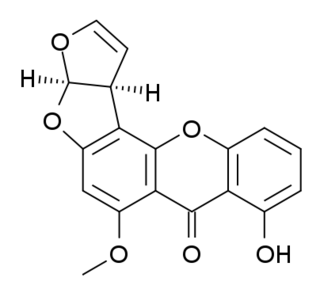
Sterigmatocystin is a polyketide mycotoxin produced by certain species of Aspergillus. The toxin is naturally found in some cheeses.

Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.

Aflatoxin B1 is an aflatoxin produced by Aspergillus flavus and A. parasiticus. It is a very potent carcinogen with a TD50 3.2 μg/kg/day in rats. This carcinogenic potency varies across species with some, such as rats and monkeys, seemingly much more susceptible than others. Aflatoxin B1 is a common contaminant in a variety of foods including peanuts, cottonseed meal, corn, and other grains; as well as animal feeds. Aflatoxin B1 is considered the most toxic aflatoxin and it is highly implicated in hepatocellular carcinoma (HCC) in humans. In animals, aflatoxin B1 has also been shown to be mutagenic, teratogenic, and to cause immunosuppression. Several sampling and analytical methods including thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), mass spectrometry, and enzyme-linked immunosorbent assay (ELISA), among others, have been used to test for aflatoxin B1 contamination in foods. According to the Food and Agriculture Organization (FAO), a division of the United Nations, the worldwide maximum tolerated levels of aflatoxin B1 was reported to be in the range of 1–20 μg/kg (or .001 ppm - 1 part-per-billion) in food, and 5–50 μg/kg (.005 ppm) in dietary cattle feed in 2003.
Aspergillus ochraceus is a mold species in the genus Aspergillus known to produce the toxin ochratoxin A, one of the most abundant food-contaminating mycotoxins, and citrinin. It also produces the dihydroisocoumarin mellein. It is a filamentous fungus in nature and has characteristic biseriate conidiophores. Traditionally a soil fungus, has now began to adapt to varied ecological niches, like agricultural commodities, farmed animal and marine species. In humans and animals the consumption of this fungus produces chronic neurotoxic, immunosuppressive, genotoxic, carcinogenic and teratogenic effects. Its airborne spores are one of the potential causes of asthma in children and lung diseases in humans. The pig and chicken populations in the farms are the most affected by this fungus and its mycotoxins. Certain fungicides like mancozeb, copper oxychloride, and sulfur have inhibitory effects on the growth of this fungus and its mycotoxin producing capacities.
Aspergillus penicillioides is a species of fungus in the genus Aspergillus, and is among the most xerophilic fungi.

Aspergillus versicolor is a slow-growing filamentous fungus commonly found in damp indoor environments and on food products. It has a characteristic musty odor associated with moldy homes and is a major producer of the hepatotoxic and carcinogenic mycotoxin sterigmatocystin. Like other Aspergillus species, A. versicolor is an eye, nose, and throat irritant.
Aspergillus unguis is a species of fungus in the genus Aspergillus, and the asexual state (anamorph) of Emericella unguis. Aspergillus unguis is a filamentous soil-borne fungus found on decomposing plant matter and other moist substrates including with building materials and household dust. Aspergillus unguis occurs mainly in tropical and subtropical soils but has also been isolated from various marine and aquatic habitats. The species was first isolated in 1935 by Weill and L. Gaudin. Historically, A. unguis was assigned to the A. nidulans group, a common group of soil-borne fungi due to the resemblance of its ascospores and cleistothecia to those of Emericella nidulans. Aspergillus unguis is distinctive, however, in possessing spicular hyphae. A number of synonyms have been collapsed into this species, including Sterigmatocystis unguis, Aspergillus laokiashanensis and Aspergillus mellinus.
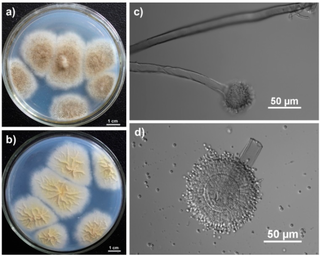
Aspergillus tubingensis is a darkly pigmented species of fungus in the genus Aspergillus section Nigri. It is often confused with Aspergillus niger due to their similar morphology and habitat. A. tubingensis is often involved in food spoilage of fruits and wheat, and industrial fermentation. This species is a rare agent of opportunistic infection.
Aspergillus leporis is an anamorph species of fungus in the genus Aspergillus. It is from the Flavi section. The species was first described in 1979. It has been isolated from the dung of Lepus townsendii. Aspergillus leporis produces leporin A and leporin B. It has also been reported to produce antibiotic Y, kojic acid, and pseurotin.
Aspergillus nomius is a species of fungus in the genus Aspergillus. It is from the Flavi section. The species was first described in 1987. It has been reported to produce aflatoxin B1, aflatoxin B2, aflatoxin G1, aflatoxin G2, aspergillic acid, kojic acid, nominine, paspaline, pseurotin, and tenuazonic acid. A. nomius has been identified as the cause of human infections.
Aspergillus wentii is an asexual, filamentous, endosymbiotic fungus belonging to the mold genus, Aspergillus. It is a common soil fungus with a cosmopolitan distribution, although it is primarily found in subtropical regions. Found on a variety of organic materials, A. wentii is known to colonize corn, cereals, moist grains, peanuts and other ground nut crops. It is also used in the manufacture of biodiesel from lipids and is known for its ability to produce enzymes used in the food industry.
John Ingram Pitt was an Australian mycologist, known as a leading expert on the role of fungi in food spoilage. He gained an international reputation as a pioneering researcher on the ecology of spoilage moulds in extreme environments and of dried fruits and other dried foodstuffs.













