
Ricinus communis, the castor bean or castor oil plant, is a species of perennial flowering plant in the spurge family, Euphorbiaceae. It is the sole species in the monotypic genus, Ricinus, and subtribe, Ricininae. The evolution of castor and its relation to other species are currently being studied using modern genetic tools. It reproduces with a mixed pollination system which favors selfing by geitonogamy but at the same time can be an out-crosser by anemophily or entomophily.

In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.
Saponins, also selectively referred to as triterpene glycosides, are bitter-tasting usually toxic plant-derived organic chemicals that have a foamy quality when agitated in water. They are widely distributed but found particularly in soapwort, a flowering plant, the soapbark tree and soybeans. They are used in soaps, medicines, fire extinguishers, speciously as dietary supplements, for synthesis of steroids, and in carbonated beverages. Saponins are both water and fat soluble, which gives them their useful soap properties. Some examples of these chemicals are glycyrrhizin and quillaia, a bark extract used in beverages.

Mimosa tenuiflora, syn. Mimosa hostilis, also known as jurema preta, calumbi (Brazil), tepezcohuite (México), carbonal, cabrera, jurema, black jurema, and binho de jurema, is a perennial tree or shrub native to the northeastern region of Brazil and found as far north as southern Mexico, and the following countries: El Salvador, Honduras, Panama, Colombia and Venezuela. It is most often found in lower altitudes, but it can be found as high as 1,000 m (3,300 ft).
The name Catuaba is used for the infusions of the bark of a number of trees native to Brazil. The most widely used barks are derived from the trees Trichilia catigua and Erythroxylum vaccinifolium. Other catuaba preparations use the bark of trees from the following genera or families: Anemopaegma, Ilex, Micropholis, Phyllanthus, Secondatia, Tetragastris and species from the Myrtaceae.

Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids.

Senegalia rugata is a spiny climbing shrub native to China and tropical Asia, common in the warm plains of central and south India. It is renowned as a raw material for shampoo, and the leaves and young shoots are often eaten. Archaeobotanical evidence shows its use for hair care in the pre-Harrapan levels of Banawali, some 4500–4300 years ago.

Adenanthera pavonina is a perennial and non-climbing species of leguminous tree. Its uses include food and drink, traditional medicine, and timber.

Dacryodes edulis is a fruit tree in the Burseraceae family native to Africa. Its various regional names include safou, plum (Cameroon), atanga, ube, elumi (Nigeria), African pear, bush pear, African plum, nsafu, bush butter tree, or butterfruit.
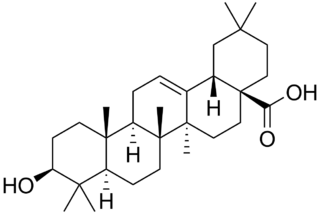
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins.

Ximenia americana, commonly known as tallow wood, hog plum, yellow plum, sea lemon, or pi'ut (Chamorro), is bush-forming shrub/small tree; a species from the Ximenia genus in the Olacaceae family. It is mainly found in the tropics, ranging from Africa, India and southeast Asia, to Australia, New Zealand, Pacific Islands, West Indies, Central, North and South America. It is especially common in Africa and South America. It is not domesticated so it is only found occurring in the wild.

Prenylated flavonoids or prenylflavonoids are a sub-class of flavonoids. They are widely distributed throughout the plant kingdom. Some are known to have phytoestrogenic or antioxidant properties. They are given in the list of adaptogens in herbalism. Chemically they have a prenyl group attached to their flavonoid backbone. It is usually assumed that the addition of hydrophobic prenyl groups facilitate attachment to cell membranes. Prenylation may increase the potential activity of its original flavonoid.

Hederagenin is a triterpenoid which is a chemical constituent of the Hedera helix plant.

Taxodone is a naturally occurring diterpenoid found in Taxodium distichum, Rosmarinus officinalis (rosemary), several salvia species and other plants, along with its oxidized rearrangement product, taxodione. Taxodone and taxodione exhibit anticancer, antibacterial, antioxidant, antifungal, insecticide, and antifeedant activities.

A quinone methide is a type of conjugated organic compound that contain a cyclohexadiene with a carbonyl and an exocyclic methylidene or extended alkene unit. It is analogous to a quinone, but having one of the double bonded oxygens replaced with a carbon. The carbonyl and methylidene are usually oriented either ortho or para to each other. There are some examples of transient synthetic meta quinone methides.
Droplet countercurrent chromatography was introduced in 1970 by Tanimura, Pisano, Ito, and Bowman. DCCC is considered to be a form of liquid-liquid separation, which includes countercurrent distribution and countercurrent chromatography, that employs a liquid stationary phase held in a collection of vertical glass columns connected in series. The mobile phase passes through the columns in the form of droplets. The DCCC apparatus may be run with the lower phase stationary and the upper phase being introduced to the bottom of each column. Or it may be run with the upper phase stationary and the lower phase being introduced from the top of the column. In both cases, the work of gravity is allowed influence the two immiscible liquids of different densities to form the signature droplets that rise or descend through the column. The mobile phase is pumped at a rate that will allow droplets to form that maximize the mass transfer of a compound between the upper and lower phases. Compounds that are more soluble in the upper phase will travel quickly through the column, while compounds that are more soluble in the stationary phase will linger. Separation occurs because different compounds distribute differently, in a ratio called the partition coefficient, between the two phases.

Phlomoides tuberosa, the sage-leaf mullein, is a perennial herbaceous flowering plant in the family Lamiaceae, native to China, Kazakhstan, Kyrgyzstan, Mongolia, Russia; SW Asia and Europe. Enlarged, tuberous roots give rise to erect stems to 150 cm bearing purple-red flowers.

Chuquiraga spinosa, common name huamanpinta in Spanish, is a species of flowering plant of the family Asteraceae. Native to Perú and Bolivia, it is used in traditional medicine for its anti-inflammatory properties.

Dictyota dichotoma is a species of Brown algae found in the temperate western and eastern Atlantic Ocean, the Mediterranean Sea, the Black Sea, the Red Sea and the western Indian Ocean.
Manilkara obovata is small to large sized evergreen tree within the Sapotaceae family. Its timber is sold under the name Nkunya in Uganda. The species has a wide distribution from Sierra Leone in West Africa moving east to Uganda in Eastern Africa and southwards to Zambia. It is also considered a variable species having different ecotypes.

















