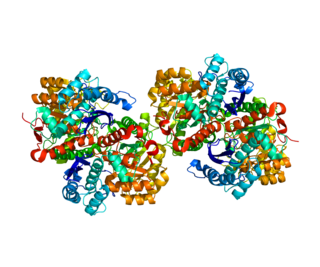In anatomy, a crystallin is a water-soluble structural protein found in the lens and the cornea of the eye accounting for the transparency of the structure. It has also been identified in other places such as the heart, and in aggressive breast cancer tumors. The physical origins of eye lens transparency and its relationship to cataract are an active area of research. Since it has been shown that lens injury may promote nerve regeneration, crystallin has been an area of neural research. So far, it has been demonstrated that crystallin β b2 (crybb2) may be a neurite-promoting factor.

Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate Branchiostoma.

Desmin is a protein that in humans is encoded by the DES gene. Desmin is a muscle-specific, type III intermediate filament that integrates the sarcolemma, Z disk, and nuclear membrane in sarcomeres and regulates sarcomere architecture.
MYH7 is a gene encoding a myosin heavy chain beta (MHC-β) isoform expressed primarily in the heart, but also in skeletal muscles. This isoform is distinct from the fast isoform of cardiac myosin heavy chain, MYH6, referred to as MHC-α. MHC-β is the major protein comprising the thick filament that forms the sarcomeres in cardiac muscle and plays a major role in cardiac muscle contraction.
Heat shock protein 27 (Hsp27) also known as heat shock protein beta-1 (HSPB1) is a protein that in humans is encoded by the HSPB1 gene.
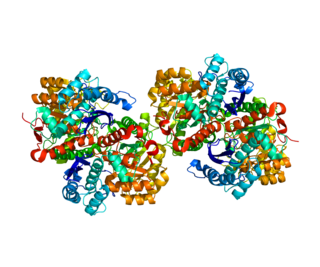
Enolase 1 (ENO1), more commonly known as alpha-enolase, is a glycolytic enzyme expressed in most tissues, one of the isozymes of enolase. Each isoenzyme is a homodimer composed of 2 alpha, 2 gamma, or 2 beta subunits, and functions as a glycolytic enzyme. Alpha-enolase, in addition, functions as a structural lens protein (tau-crystallin) in the monomeric form. Alternative splicing of this gene results in a shorter isoform that has been shown to bind to the c-myc promoter and function as a tumor suppressor. Several pseudogenes have been identified, including one on the long arm of chromosome 1. Alpha-enolase has also been identified as an autoantigen in Hashimoto encephalopathy.

Heat shock protein HSP 90-beta also called HSP90beta is a protein that in humans is encoded by the HSP90AB1 gene.
Gamma-crystallin D is a protein that in humans is encoded by the CRYGD gene.

Hsc70-interacting protein also known as suppression of tumorigenicity 13 (ST13) is a protein that in humans is encoded by the ST13 gene.

Crystallin, gamma C, also known as CRYGC, is a protein which in humans is encoded by the CRYGC gene.

Myosin heavy chain, α isoform (MHC-α) is a protein that in humans is encoded by the MYH6 gene. This isoform is distinct from the ventricular/slow myosin heavy chain isoform, MYH7, referred to as MHC-β. MHC-α isoform is expressed predominantly in human cardiac atria, exhibiting only minor expression in human cardiac ventricles. It is the major protein comprising the cardiac muscle thick filament, and functions in cardiac muscle contraction. Mutations in MYH6 have been associated with late-onset hypertrophic cardiomyopathy, atrial septal defects and sick sinus syndrome.

Gamma-crystallin B is a protein that in humans is encoded by the CRYGB gene.

Myosin essential light chain (ELC), ventricular/cardiac isoform is a protein that in humans is encoded by the MYL3 gene. This cardiac ventricular/slow skeletal ELC isoform is distinct from that expressed in fast skeletal muscle (MYL1) and cardiac atrial muscle (MYL4). Ventricular ELC is part of the myosin molecule and is important in modulating cardiac muscle contraction.

LIM domain binding 3 (LDB3), also known as Z-band alternatively spliced PDZ-motif (ZASP), is a protein which in humans is encoded by the LDB3 gene. ZASP belongs to the Enigma subfamily of proteins and stabilizes the sarcomere during contraction, through interactions with actin in cardiac and skeletal muscles. Mutations in the ZASP gene has been associated with several muscular diseases.

Heat shock protein beta-6 (HSPB6) is a protein that in humans is encoded by the HSPB6 gene.

Alpha-crystallin A chain is a protein that in humans is encoded by the CRYAA gene.

Protein moonlighting is a phenomenon by which a protein can perform more than one function. It is an excellent example of gene sharing.

Heat shock protein beta-3 (HspB3) also known as heat shock 27kDa protein 3 is a protein that in humans is encoded by the HSPB3 gene.

NADH:ubiquinone oxidoreductase complex assembly factor 2 (NDUFAF2), also known as B17.2L or NDUFA12L, is a protein that in humans is encoded by the NDUFAF2, or B17.2L, gene. The NDUFAF2 protein is a chaperone involved in the assembly of NADH dehydrogenase (ubiquinone) also known as complex I, which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain. Mutations in this gene have been associated with progressive encephalopathy and Leigh disease resulting from mitochondrial complex I deficiency.

Heat Shock Protein Family B (small) member 7 (HSPB7) in humans is a protein encoded by a gene of the same name with four exons that is located on chromosome 1p36.13.,. HSPB7 contains 170 amino acids and has a molecular weight of 18,611Da. HSPB7 is a member of human small heat shock protein (HSPB) family, which contains eleven family members of chaperone proteins. HSPB7 and its gene pair SRARP are located 5 kb apart on the opposite strands of chromosome 1p36.13.