
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis.
Heat shock proteins (HSP) are a family of proteins produced by cells in response to exposure to stressful conditions. They were first described in relation to heat shock, but are now known to also be expressed during other stresses including exposure to cold, UV light and during wound healing or tissue remodeling. Many members of this group perform chaperone functions by stabilizing new proteins to ensure correct folding or by helping to refold proteins that were damaged by the cell stress. This increase in expression is transcriptionally regulated. The dramatic upregulation of the heat shock proteins is a key part of the heat shock response and is induced primarily by heat shock factor (HSF). HSPs are found in virtually all living organisms, from bacteria to humans.
In anatomy, a crystallin is a water-soluble structural protein found in the lens and the cornea of the eye accounting for the transparency of the structure. It has also been identified in other places such as the heart, and in aggressive breast cancer tumors. Since it has been shown that lens injury may promote nerve regeneration, crystallin has been an area of neural research. So far, it has been demonstrated that crystallin β b2 (crybb2) may be a neurite-promoting factor.
The 70 kilodalton heat shock proteins are a family of conserved ubiquitously expressed heat shock proteins. Proteins with similar structure exist in virtually all living organisms. Intracellularly localized Hsp70s are an important part of the cell's machinery for protein folding, performing chaperoning functions, and helping to protect cells from the adverse effects of physiological stresses. Additionally, membrane-bound Hsp70s have been identified as a potential target for cancer therapies and their extracellularly localized counterparts have been identified as having both membrane-bound and membrane-free structures.
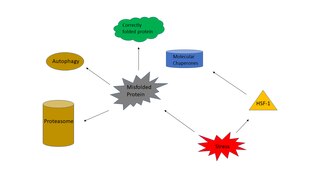
The heat shock response (HSR) is a cell stress response that increases the number of molecular chaperones to combat the negative effects on proteins caused by stressors such as increased temperatures, oxidative stress, and heavy metals. In a normal cell, proteostasis must be maintained because proteins are the main functional units of the cell. Many proteins take on a defined configuration in a process known as protein folding in order to perform their biological functions. If these structures are altered, critical processes could be affected, leading to cell damage or death. The heat shock response can be employed under stress to induce the expression of heat shock proteins (HSP), many of which are molecular chaperones, that help prevent or reverse protein misfolding and provide an environment for proper folding.

Heat shock 70 kDa protein 8 also known as heat shock cognate 71 kDa protein or Hsc70 or Hsp73 is a heat shock protein that in humans is encoded by the HSPA8 gene on chromosome 11. As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. Its functions contribute to biological processes including signal transduction, apoptosis, autophagy, protein homeostasis, and cell growth and differentiation. It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence, and aging.

Heat shock 70 kDa protein 1, also termed Hsp72, is a protein that in humans is encoded by the HSPA1A gene. As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. In addition, Hsp72 also facilitates DNA repair. Its functions contribute to biological processes including signal transduction, apoptosis, protein homeostasis, and cell growth and differentiation. It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence and aging, and inflammatory diseases such as Diabetes mellitus type 2 and rheumatoid arthritis.

Heat shock protein HSP 90-alpha is a protein that in humans is encoded by the HSP90AA1 gene.

Heat shock factor 1 (HSF1) is a protein that in humans is encoded by the HSF1 gene. HSF1 is highly conserved in eukaryotes and is the primary mediator of transcriptional responses to proteotoxic stress with important roles in non-stress regulation such as development and metabolism.

Alpha-crystallin B chain is a protein that in humans is encoded by the CRYAB gene. It is part of the small heat shock protein family and functions as molecular chaperone that primarily binds misfolded proteins to prevent protein aggregation, as well as inhibit apoptosis and contribute to intracellular architecture. Post-translational modifications decrease the ability to chaperone. Mutations in CRYAB cause different cardiomyopathies, skeletal myopathies mainly myofibrillar myopathy, and also cataracts. In addition, defects in this gene/protein have been associated with cancer and neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease.

Heat shock protein HSP 90-beta also called HSP90beta is a protein that in humans is encoded by the HSP90AB1 gene.

Heat shock protein beta-8 is a protein that in humans is encoded by the HSPB8 gene.

Heat shock protein beta-2 is a protein that in humans is encoded by the HSPB2 gene.

Heat shock 70 kDa protein 1L is a protein that in humans is encoded by the HSPA1L gene on chromosome 6. As a member of the heat shock protein 70 (Hsp70) family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. Its functions contribute to biological processes including signal transduction, apoptosis, protein homeostasis, and cell growth and differentiation. It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence and aging, and Graft-versus-host disease.

Heat shock protein beta-6 (HSPB6) is a protein that in humans is encoded by the HSPB6 gene.

Alpha-crystallin A chain is a protein that in humans is encoded by the CRYAA gene.
The heat shock protein Hsp20 family, also known as small heat shock proteins (sHSPs), is a family of heat shock proteins.

Protein moonlighting is a phenomenon by which a protein can perform more than one function. It is an excellent example of gene sharing.

Heat shock protein beta-3 (HspB3) also known as heat shock 27kDa protein 3 is a protein that in humans is encoded by the HSPB3 gene.
Hsp104 is a heat-shock protein. It is known to reverse toxicity of mutant α-synuclein, TDP-43, FUS, and TAF15 in yeast cells. Conserved in prokaryotes (ClpB), fungi, plants and aswell as animal mitochondria, there is yet to see hsp104 in multicellular animals. Hsp104 is classified as a. AAA+ ATPases and a subgroup of Hsp100/Clp, because of the usage of Atp hydrolysis for structural modulation of other proteins. Hsp104 is not needed for normal cell growth but when exposed to stress there is an increase amount. Removing the aggregates without the hsp104 is insufficient there highlighting the importance of this heat shock protein and its interactions.




















