
Interferons are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.

Interferon gamma is a dimerized soluble cytokine that is the only member of the type II class of interferons. The existence of this interferon, which early in its history was known as immune interferon, was described by E. F. Wheelock as a product of human leukocytes stimulated with phytohemagglutinin, and by others as a product of antigen-stimulated lymphocytes. It was also shown to be produced in human lymphocytes. or tuberculin-sensitized mouse peritoneal lymphocytes challenged with Mantoux test (PPD); the resulting supernatants were shown to inhibit growth of vesicular stomatitis virus. Those reports also contained the basic observation underlying the now widely employed interferon gamma release assay used to test for tuberculosis. In humans, the IFNG protein is encoded by the IFNG gene.
Interferon tau is a Type I interferon made of a single chain of amino acids. IFN-τ was first discovered in ruminants as the signal for the maternal recognition of pregnancy and originally named ovine trophoblast protein-1 (oTP-1). It has many physiological functions in the mammalian uterus, and also has anti-inflammatory effect that aids in the protection of the semi-allogeneic conceptus trophectoderm from the maternal immune system.

Interferon regulatory factors (IRF) are proteins which regulate transcription of interferons. Interferon regulatory factors contain a conserved N-terminal region of about 120 amino acids, which folds into a structure that binds specifically to the IRF-element (IRF-E) motifs, which is located upstream of the interferon genes. Some viruses have evolved defense mechanisms that regulate and interfere with IRF functions to escape the host immune system. For instance, the remaining parts of the interferon regulatory factor sequence vary depending on the precise function of the protein. The Kaposi sarcoma herpesvirus, KSHV, is a cancer virus that encodes four different IRF-like genes; including vIRF1, which is a transforming oncoprotein that inhibits type 1 interferon activity. In addition, the expression of IRF genes is under epigenetic regulation by promoter DNA methylation.

Interleukin-29 (IL-29) is a cytokine and it belongs to type III interferons group, also termed interferons λ (IFN-λ). IL-29 plays an important role in the immune response against pathogenes and especially against viruses by mechanisms similar to type I interferons, but targeting primarily cells of epithelial origin and hepatocytes.
The type III interferon group is a group of anti-viral cytokines, that consists of four IFN-λ (lambda) molecules called IFN-λ1, IFN-λ2, IFN-λ3, and IFN-λ4. They were discovered in 2003. Their function is similar to that of type I interferons, but is less intense and serves mostly as a first-line defense against viruses in the epithelium.

Signal transducer and activator of transcription 1 (STAT1) is a transcription factor which in humans is encoded by the STAT1 gene. It is a member of the STAT protein family.

Non-receptor tyrosine-protein kinase TYK2 is an enzyme that in humans is encoded by the TYK2 gene.

Signal transducer and activator of transcription 4 (STAT4) is a transcription factor belonging to the STAT protein family, composed of STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, STAT6. STAT proteins are key activators of gene transcription which bind to DNA in response to cytokine gradient. STAT proteins are a common part of Janus kinase (JAK)- signalling pathways, activated by cytokines.STAT4 is required for the development of Th1 cells from naive CD4+ T cells and IFN-γ production in response to IL-12. There are two known STAT4 transcripts, STAT4α and STAT4β, differing in the levels of interferon-gamma production downstream.

Signal transducer and activator of transcription 2 is a protein that in humans is encoded by the STAT2 gene. It is a member of the STAT protein family. This protein is critical to the biological response of type I interferons (IFNs). It functions as a transcription factor downstream of type I interferons. STAT2 sequence identity between mouse and human is only 68%.

RIG-I is a cytosolic pattern recognition receptor (PRR) that can mediate induction of a type-I interferon (IFN1) response. RIG-I is an essential molecule in the innate immune system for recognizing cells that have been infected with a virus. These viruses can include West Nile virus, Japanese Encephalitis virus, influenza A, Sendai virus, flavivirus, and coronaviruses.
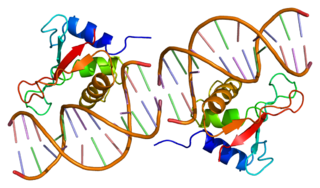
Interferon regulatory factor 1 is a protein that in humans is encoded by the IRF1 gene.

Mitochondrial antiviral-signaling protein (MAVS) is a protein that is essential for antiviral innate immunity. MAVS is located in the outer membrane of the mitochondria, peroxisomes, and mitochondrial-associated endoplasmic reticulum membrane (MAM). Upon viral infection, a group of cytosolic proteins will detect the presence of the virus and bind to MAVS, thereby activating MAVS. The activation of MAVS leads the virally infected cell to secrete cytokines. This induces an immune response which kills the host's virally infected cells, resulting in clearance of the virus.

Ubiquitin/ISG15-conjugating enzyme E2 L6 is a protein that in humans is encoded by the UBE2L6 gene.

Ubiquitin specific peptidase 18 (USP18), also known as UBP43, is a type I interferon receptor repressor and an isopeptidase. In humans, it is encoded by the USP18 gene. USP18 is induced by the immune response to type I and III interferons, and serves as a negative regulator of type I interferon, but not type III interferon. Loss of USP18 results in increased responsiveness to type I interferons and life-threatening autoinflammatory disease in humans due to the negative regulatory function of USP18 in interferon signal transduction. Independent of this activity, USP18 is also a member of the deubiquitinating protease family of enzymes. It is known to remove ISG15 conjugates from a broad range of protein substrates, a process known as deISGylation.

Ubiquitin-like modifier-activating enzyme 7 is a protein that in humans is encoded by the UBA7 gene.

Ubiquitin-conjugating enzyme E2 E2 is a protein that in humans is encoded by the UBE2E2 gene.

E3 ISG15–protein ligase HERC5 is an enzyme that in humans is encoded by the HERC5 gene.

The Interleukin-1 family is a group of 11 cytokines that plays a central role in the regulation of immune and inflammatory responses to infections or sterile insults.
An interferon-stimulated gene (ISG) is a gene that can be expressed in response to stimulation by interferon. Interferons bind to receptors on the surface of a cell, initiating protein signaling pathways within the cell. This interaction leads to the expression of a subset of genes involved in the innate immune system response. ISGs are commonly expressed in response to viral infection, but also during bacterial infection and in the presence of parasites. It's currently estimated that 10% of the human genome is regulated by interferons (IFNs). Interferon stimulated genes can act as an initial response to pathogen invasion, slowing down viral replication and increasing expression of immune signaling complexes. There are three known types of interferon. With approximately 450 genes highly expressed in response to interferon type I. Type I interferon consists of INF-α, INF-β, INF-ω and is expressed in response to viral infection. ISGs induced by type I interferon are associated with viral replication suppression and increase expression of immune signaling proteins. Type II interferon consists only of INF-γ and is associated with controlling intracellular pathogens and tumor suppressor genes. Type III interferon consists of INF-λ and is associated with viral immune response and is key in anti-fungal neutrophil response.
























