| HSPA4 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | HSPA4 , APG-2, HEL-S-5a, HS24/P52, HSPH2, RY, hsp70, hsp70RY, heat shock protein family A (Hsp70) member 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 601113; MGI: 1342292; HomoloGene: 1624; GeneCards: HSPA4; OMA:HSPA4 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
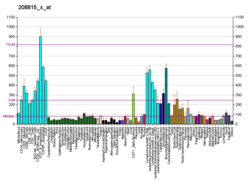
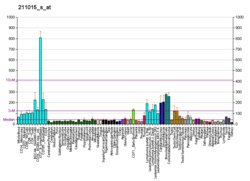
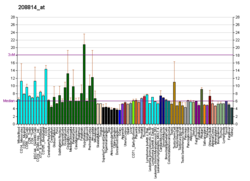
Heat shock 70 kDa protein 4 is a protein that in humans is encoded by the HSPA4 gene. [5] [6]
Contents
The protein encoded by this gene was originally suggested to be a member of the heat shock protein 70 family. [5] However it is now known that human HSPA4 is an equivalent to mouse the Apg-2 protein and is a member of the Hsp110 family. [7]