Related Research Articles
Heat shock proteins (HSP) are a family of proteins produced by cells in response to exposure to stressful conditions. They were first described in relation to heat shock, but are now known to also be expressed during other stresses including exposure to cold, UV light and during wound healing or tissue remodeling. Many members of this group perform chaperone functions by stabilizing new proteins to ensure correct folding or by helping to refold proteins that were damaged by the cell stress. This increase in expression is transcriptionally regulated. The dramatic upregulation of the heat shock proteins is a key part of the heat shock response and is induced primarily by heat shock factor (HSF). HSPs are found in virtually all living organisms, from bacteria to humans.
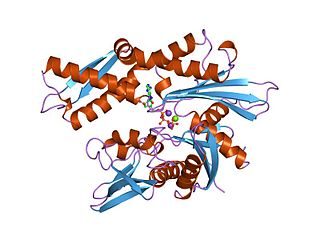
The 70 kilodalton heat shock proteins are a family of conserved ubiquitously expressed heat shock proteins. Proteins with similar structure exist in virtually all living organisms. Intracellularly localized Hsp70s are an important part of the cell's machinery for protein folding, performing chaperoning functions, and helping to protect cells from the adverse effects of physiological stresses. Additionally, membrane-bound Hsp70s have been identified as a potential target for cancer therapies and their extracellularly localized counterparts have been identified as having both membrane-bound and membrane-free structures.
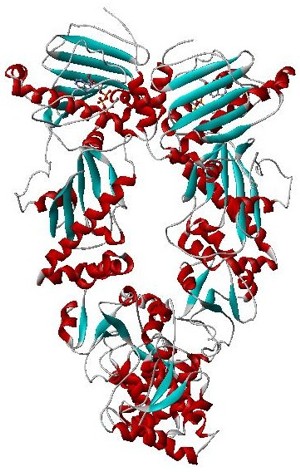
Hsp90 is a chaperone protein that assists other proteins to fold properly, stabilizes proteins against heat stress, and aids in protein degradation. It also stabilizes a number of proteins required for tumor growth, which is why Hsp90 inhibitors are investigated as anti-cancer drugs.

Hop, occasionally written HOP, is an abbreviation for Hsp70-Hsp90 Organizing Protein. It functions as a co-chaperone which reversibly links together the protein chaperones Hsp70 and Hsp90.

Heat shock 70 kDa protein 8 also known as heat shock cognate 71 kDa protein or Hsc70 or Hsp73 is a heat shock protein that in humans is encoded by the HSPA8 gene on chromosome 11. As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. Its functions contribute to biological processes including signal transduction, apoptosis, autophagy, protein homeostasis, and cell growth and differentiation. It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence, and aging.

FK506-binding protein 4 is a protein that in humans is encoded by the FKBP4 gene.

Heat shock 70 kDa protein 1, also termed Hsp72, is a protein that in humans is encoded by the HSPA1A gene. As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. In addition, Hsp72 also facilitates DNA repair. Its functions contribute to biological processes including signal transduction, apoptosis, protein homeostasis, and cell growth and differentiation. It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence and aging, and inflammatory diseases such as Diabetes mellitus type 2 and rheumatoid arthritis.

Heat shock protein HSP 90-alpha is a protein that in humans is encoded by the HSP90AA1 gene.

Human gene HSPA1B is an intron-less gene which encodes for the heat shock protein HSP70-2, a member of the Hsp70 family of proteins. The gene is located in the major histocompatibility complex, on the short arm of chromosome 6, in a cluster with two paralogous genes, HSPA1A and HSPA1L. HSPA1A and HSPA1B produce nearly identical proteins because the few differences in their DNA sequences are almost exclusively synonymous substitutions or in the three prime untranslated region, heat shock 70kDa protein 1A, from HSPA1A, and heat shock 70kDa protein 1B, from HSPA1B. A third, more modified paralog to these genes exists in the same region, HSPA1L, which shares a 90% homology with the other two.

Heat shock factor 1 (HSF1) is a protein that in humans is encoded by the HSF1 gene. HSF1 is highly conserved in eukaryotes and is the primary mediator of transcriptional responses to proteotoxic stress with important roles in non-stress regulation such as development and metabolism.

Heat shock protein 90kDa beta member 1 (HSP90B1), known also as endoplasmin, gp96, grp94, or ERp99, is a chaperone protein that in humans is encoded by the HSP90B1 gene.
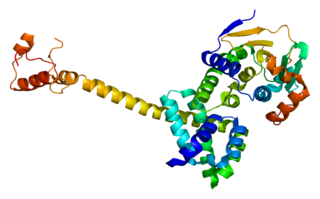
Hsp90 co-chaperone Cdc37 is a protein that in humans is encoded by the CDC37 gene. This protein is highly similar to Cdc 37, a cell division cycle control protein of Saccharomyces cerevisiae. This protein is a HSP90 Co-chaperone with specific function in cell signal transduction. It has been shown to form complex with Hsp90 and a variety of protein kinases including CDK4, CDK6, SRC, RAF1, MOK, as well as eIF-2 alpha kinases. It is thought to play a critical role in directing Hsp90 to its target kinases.

Heat shock protein HSP 90-beta also called HSP90beta is a protein that in humans is encoded by the HSP90AB1 gene.

DnaJ homolog subfamily B member 1 is a protein that in humans is encoded by the DNAJB1 gene.

FK506 binding protein 5, also known as FKBP5, is a protein which in humans is encoded by the FKBP5 gene.

DnaJ homolog subfamily A member 1 is a protein that in humans is encoded by the DNAJA1 gene.

Heat shock 70 kDa protein 14 also known as HSP70-like protein 1 or heat shock protein HSP60 is a protein that in humans is encoded by the HSPA14 gene.

Heat shock protein 75 kDa, mitochondrial is a protein that in humans is encoded by the TRAP1 gene.
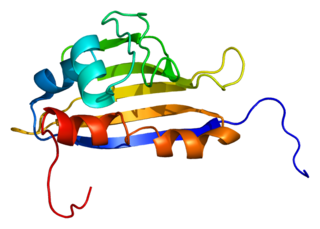
Activator of 90 kDa heat shock protein ATPase homolog 1 is an enzyme that in humans is encoded by the AHSA1 gene.
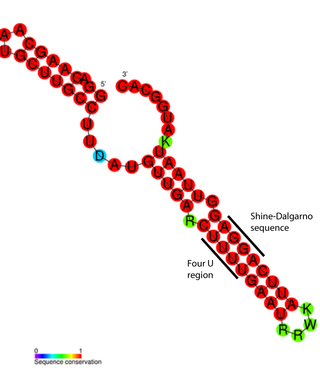
FourU thermometers are a class of non-coding RNA thermometers found in Salmonella. They are named 'FourU' due to the four highly conserved uridine nucleotides found directly opposite the Shine-Dalgarno sequence on hairpin II (pictured). RNA thermometers such as FourU control regulation of temperature via heat shock proteins in many prokaryotes. FourU thermometers are relatively small RNA molecules, only 57 nucleotides in length, and have a simple two-hairpin structure.
References
- ↑ Ozawa K, Murakami Y, Eki T, Soeda E, Yokoyama K (February 1992). "Mapping of the gene family for human heat-shock protein 90 alpha to chromosomes 1, 4, 11, and 14". Genomics. 12 (2): 214–20. doi:10.1016/0888-7543(92)90368-3. PMID 1740332.
- ↑ Chen B, Piel WH, Gui L, Bruford E, Monteiro A (December 2005). "The HSP90 family of genes in the human genome: insights into their divergence and evolution". Genomics. 86 (6): 627–37. doi: 10.1016/j.ygeno.2005.08.012 . PMID 16269234.