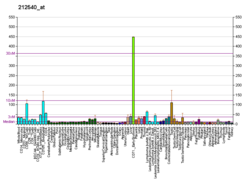
CDC34 is a gene that in humans encodes the protein Ubiquitin-conjugating enzyme E2 R1. [5] [6] [7] This protein is a member of the ubiquitin-conjugating enzyme family, which catalyzes the covalent attachment of ubiquitin to other proteins. [8]
Contents
CDC34 was originally discovered by work in baker's yeast as a gene that is essential for the cell cycle. [9] Cdc34 in yeast targets numerous substrates - notably the cyclin-dependent kinase inhibitor Sic1 [10] - for ubiquitin-mediated protein degradation. [11] CDC34 is required for ubiquitin-mediated degradation of cell cycle G1 regulators, and for the initiation of DNA replication. [7]