| Clostridium sporogenes | |
|---|---|
 | |
| Scientific classification | |
| Domain: | Bacteria |
| Kingdom: | Bacillati |
| Phylum: | Bacillota |
| Class: | Clostridia |
| Order: | Eubacteriales |
| Family: | Clostridiaceae |
| Genus: | Clostridium |
| Species: | C. sporogenes |
| Binomial name | |
| Clostridium sporogenes (Metchnikoff 1908) Bergey et al. 1923 [1] | |
Clostridium sporogenes is a species of Gram-positive bacteria that belongs to the genus Clostridium. Like other strains of Clostridium, it is an anaerobic, rod-shaped bacterium that produces oval, subterminal endospores [2] and is commonly found in soil. Unlike Clostridium botulinum , it does not produce the botulinum neurotoxins. In colonized animals, it has a mutualistic rather than pathogenic interaction with the host.
It is being investigated as a way to deliver cancer-treating drugs to tumours in patients. [3] C. sporogenes is often used as a surrogate for C. botulinum when testing the efficacy of commercial sterilisation. [4]
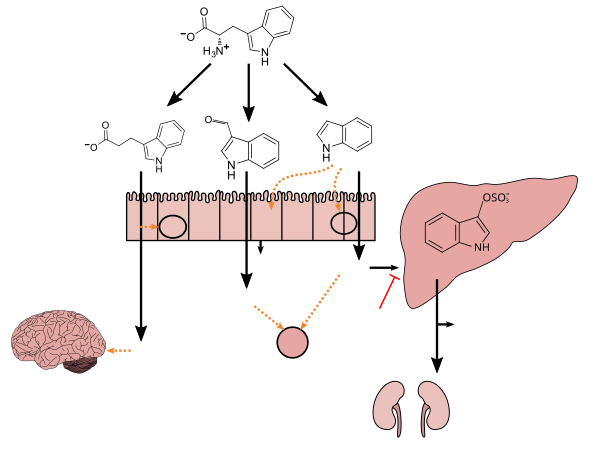
Clostridium sporogenes colonizes the human gastrointestinal tract, but is only present in a subset of the population; in the intestine, it uses tryptophan to synthesize indole and subsequently 3-indolepropionic acid (IPA) [5] – a type of auxin (plant hormone) [6] [7] – which serves as a potent neuroprotective antioxidant within the human body and brain. [5] [8] [9] [10] IPA is an even more potent scavenger of hydroxyl radicals than melatonin. [8] [9] [10] Similar to melatonin but unlike other antioxidants, it scavenges radicals without subsequently generating reactive and pro-oxidant intermediate compounds. [8] [9] [11] C. sporogenes is the only species of bacteria known to synthesize 3-indolepropionic acid in vivo at levels which are subsequently detectable in the blood stream of the host. [5] [12]
Tryptophan metabolism by human gut microbiota () |