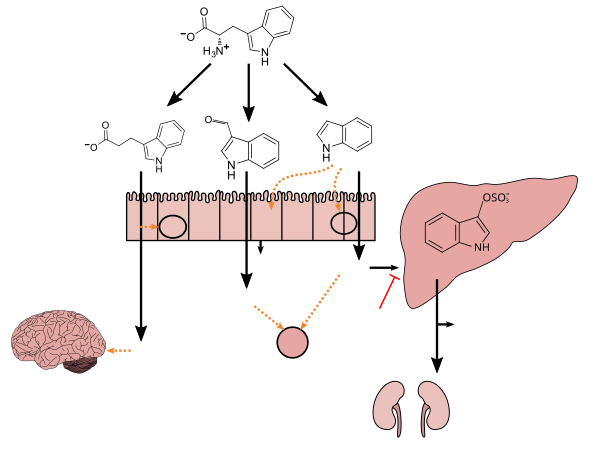
Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3 (niacin). It is encoded by the codon UGG.

The human microbiome is the aggregate of all microbiota that reside on or within human tissues and biofluids along with the corresponding anatomical sites in which they reside, including the gastrointestinal tract, skin, mammary glands, seminal fluid, uterus, ovarian follicles, lung, saliva, oral mucosa, conjunctiva, and the biliary tract. Types of human microbiota include bacteria, archaea, fungi, protists, and viruses. Though micro-animals can also live on the human body, they are typically excluded from this definition. In the context of genomics, the term human microbiome is sometimes used to refer to the collective genomes of resident microorganisms; however, the term human metagenome has the same meaning.

Melatonin, an indoleamine, is a natural compound produced by various organisms, including bacteria and eukaryotes. Its discovery in 1958 by Aaron B. Lerner and colleagues stemmed from the isolation of a substance from the pineal gland of cows that could induce skin lightening in common frogs. This compound was later identified as a hormone secreted in the brain during the night, playing a crucial role in regulating the sleep-wake cycle, also known as the circadian rhythm, in vertebrates.

Tryptamine is an indolamine metabolite of the essential amino acid tryptophan. The chemical structure is defined by an indole—a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon. The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others.
Skatole or 3-methylindole is an organic compound belonging to the indole family. It occurs naturally in the feces of mammals and birds and is the primary contributor to fecal odor. In low concentrations, it has a flowery smell and is found in several flowers and essential oils, including those of orange blossoms, jasmine, and Ziziphus mauritiana. It has also been identified in certain cannabis varieties.
The Pictet–Spengler reaction is a chemical reaction in which a β-arylethylamine undergoes condensation with an aldehyde or ketone followed by ring closure. The reaction was first discovered in 1911 by Amé Pictet and Theodor Spengler. Traditionally, an acidic catalyst in protic solvent was employed with heating; however, the reaction has been shown to work in aprotic media in superior yields and sometimes without acid catalysis. The Pictet–Spengler reaction can be considered a special case of the Mannich reaction, which follows a similar reaction pathway. The driving force for this reaction is the electrophilicity of the iminium ion generated from the condensation of the aldehyde and amine under acid conditions. This explains the need for an acid catalyst in most cases, as the imine is not electrophilic enough for ring closure but the iminium ion is capable of undergoing the reaction.

Indole-3-acetic acid is the most common naturally occurring plant hormone of the auxin class. It is the best known of the auxins, and has been the subject of extensive studies by plant physiologists. IAA is a derivative of indole, containing a carboxymethyl substituent. It is a colorless solid that is soluble in polar organic solvents.

Gut microbiota, gut microbiome, or gut flora are the microorganisms, including bacteria, archaea, fungi, and viruses, that live in the digestive tracts of animals. The gastrointestinal metagenome is the aggregate of all the genomes of the gut microbiota. The gut is the main location of the human microbiome. The gut microbiota has broad impacts, including effects on colonization, resistance to pathogens, maintaining the intestinal epithelium, metabolizing dietary and pharmaceutical compounds, controlling immune function, and even behavior through the gut–brain axis.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

Janthinobacterium lividum is an aerobic, Gram-negative, soil-dwelling bacterium that has a distinctive dark-violet color, due to a compound called violacein, which is produced when glycerol is metabolized as a carbon source. Violacein has antibacterial, antiviral, and antifungal properties. Its antifungal properties are of particular interest, since J. lividum is found on the skin of certain amphibians, including the red-backed salamander, where it prevents infection by the devastating chytrid fungus.

Indole is an organic compound with the formula C6H4CCNH3. Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole where one or more of the hydrogen atoms have been replaced by substituent groups. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin.

Melatonin receptor agonists are analogues of melatonin that bind to and activate the melatonin receptor. Agonists of the melatonin receptor have a number of therapeutic applications including treatment of sleep disorders and depression. The discovery and development of melatonin receptor agonists was motivated by the need for more potent analogues than melatonin, with better pharmacokinetics and longer half-lives. Melatonin receptor agonists were developed with the melatonin structure as a model.

Clostridium sporogenes is a species of Gram-positive bacteria that belongs to the genus Clostridium. Like other strains of Clostridium, it is an anaerobic, rod-shaped bacterium that produces oval, subterminal endospores and is commonly found in soil. Unlike Clostridium botulinum, it does not produce the botulinum neurotoxins. In colonized animals, it has a mutualistic rather than pathogenic interaction with the host.

The gut–brain axis is the two-way biochemical signaling that takes place between the gastrointestinal tract and the central nervous system (CNS). The term "microbiota–gut–brain axis" highlights the role of gut microbiota in these biochemical signaling. Broadly defined, the gut–brain axis includes the central nervous system, neuroendocrine system, neuroimmune systems, the hypothalamic–pituitary–adrenal axis, sympathetic and parasympathetic arms of the autonomic nervous system, the enteric nervous system, vagus nerve, and the gut microbiota.
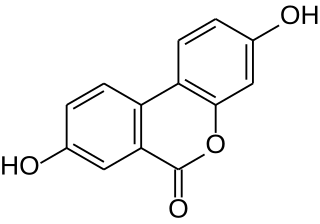
Urolithins are microflora metabolites of dietary ellagic acid derivatives, such as ellagitannins. They are produced in the gut, and found in the urine in the form of urolithin B glucuronide after absorption of ellagitannins-containing foods, such as pomegranate. During intestinal metabolism by bacteria, ellagitannins and punicalagins are converted to urolithins, which have unknown biological activity in vivo.

3-Indolepropionic acid (IPA), or indole-3-propionic acid, has been studied for its therapeutic value in the treatment of Alzheimer's disease. As of 2022 IPA shows potential in the treatment of this disease, though the therapeutic effect of IPA depends on dose and time of therapy initiation.

6-Formylindolo[3,2-b]carbazole (FICZ) is a chemical compound with the molecular formula C19H12N2O. It is a nitrogen heterocycle, having an extremely high affinity (Kd = 7 x 10−11M) for binding to the aryl hydrocarbon receptor (AHR).
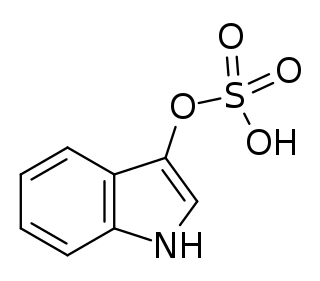
Indoxyl sulfate, also known as 3-indoxylsulfate and 3-indoxylsulfuric acid, is a metabolite of dietary L-tryptophan that acts as a cardiotoxin and uremic toxin. High concentrations of indoxyl sulfate in blood plasma are known to be associated with the development and progression of chronic kidney disease and vascular disease in humans. As a uremic toxin, it stimulates glomerular sclerosis and renal interstitial fibrosis.
Indoleacetate decarboxylase (IAD) is a glycyl radical enzyme that catalyses the decarboxylation of indoleacetate to form skatole, which is a malodorous organic compound that gives animal faeces their characteristic smell. This decarboxylation is the last step of the tryptophan fermentation in some types of anaerobic bacteria.

N1-Acetyl-5-methoxykynuramine (AMK) is a metabolite of melatonin that could improve memory by acting on the melatonin receptors. AMK is produced from the metabolization of melatonin by the kynuramine pathway in the brain. It significantly increased the phosphorylation of both ERK and CREB in the hippocampus. It also helps scavenge free radicals. AMK is highly reactive towards dioxygen (O2) radicals because of AMK's N2-amino group.