This article may be in need of reorganization to comply with Wikipedia's layout guidelines .(July 2025) |
 | |
| Names | |
|---|---|
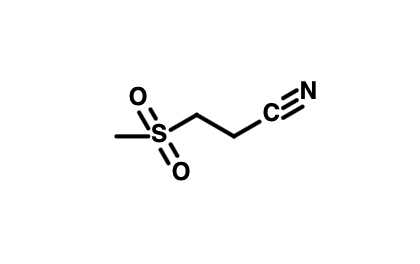
| Preferred IUPAC name 3-(Methanesulfonyl)propanenitrile | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.255.888 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C4H7NO2S | |
| Molar mass | 133.17 g·mol−1 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dapansutrile (OLT1177) is an inhibitor of the NLRP3 (nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor 3) inflammasome. [1]
Contents
- Molecular structure and properties
- Synthesis
- Dapansutrile's mechanism of action
- Dapansutrile's reaction pathways
- Summary biological effects of dapansutrile
- Pharmacokinetics
- Selectivity
- Other drug targets
- Dosages
- Pharmacodynamics
- Safety and efficacy
- Potential Therapeutic Applications
- Neurodegenerative diseases
- Inflammation
- Cardiovascular Diseases
- References

An inflammasome can be defined as an immune system receptor that induces inflammation through the activation of caspase 1 and caspase 11 when it is triggered by damaged cells, microbial pathogens, and stress. [3] NLRP3 is a canonical inflammasome. [3] The NLRP3 inflammasome comprises NLRP3, the apoptosis spec-like protein (ASC) and the caspase-1 (Figure 1). [2] The NLRP3 inflammasome forms by binding to pattern recognition receptors (PRRs) and damage associated molecular patterns damage-associated molecular patterns (DAMPS) that activate caspase 1 which then signals for the secretion of pro-inflammatory cytokines IL-1β and IL-18 resulting in pyroptosis [3] [4] [5] Constant activation of the NLRP3 inflammasome is believed to play a direct or indirect role in acute arthritis, atherosclerosis and various neurodegenerative diseases such as multiple sclerosis (MS), Alzheimer's disease (AD), and Parkinson's disease (PD). [6] [2] [7] This drug was developed by Olatec Therapeutics with the purpose of decreasing IL-1β peripheral inflammation by binding to the NLRP3 protein and inhibiting the formation of the NLRP3 inflammasome. Interestingly, dapansutrile has also been found to reduce levels of pro inflammatory cytokines IL-18 without interfering with TNF-α levels. [1] Stressed cells in the system can ignite the NLRP3 inflammasome which in turn produces the secretion inflammatory cytokines such as IL-1β and IL-18. Dapansutrile has tested in clinical trials and has been proposed as a beneficial compound for the remedy of osteoarthritis, and gouty arthritis. [1] Nevertheless, other preclinical research has proposed dapansutrile to be potentially beneficial for heart failure and multiple sclerosis. [1]