
Aralkylamine N-acetyltransferase (AANAT), also known as arylalkylamine N-acetyltransferase or serotonin N-acetyltransferase (SNAT), is an enzyme that is involved in the day/night rhythmic production of melatonin, by modification of serotonin. It is in humans encoded by the ~2.5 kb AANAT gene containing four exons, located on chromosome 17q25. The gene is translated into a 23 kDa large enzyme. It is well conserved through evolution and the human form of the protein is 80 percent identical to sheep and rat AANAT. It is an acetyl-CoA-dependent enzyme of the GCN5-related family of N-acetyltransferases (GNATs). It may contribute to multifactorial genetic diseases such as altered behavior in sleep/wake cycle and research is on-going with the aim of developing drugs that regulate AANAT function.
In enzymology, a [acyl-carrier-protein] S-acetyltransferase is an enzyme that catalyzes the reversible chemical reaction
In enzymology, a deacetylcephalosporin-C acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a deacetylvindoline O-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a homoserine O-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a salutaridinol 7-O-acetyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a serine O-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a vinorine synthase is an enzyme that catalyzes the chemical reaction

Ergocryptine is an ergopeptine and one of the ergot alkaloids. It is isolated from ergot or fermentation broth and it serves as starting material for the production of bromocriptine.
Chanoclavine-I dehydrogenase (EC 1.1.1.332, easD (gene), fgaDH (gene)) is an enzyme with systematic name chanoclavine-I:NAD+ oxidoreductase. This enzyme catalises the following chemical reaction
Festuclavine dehydrogenase (EC 1.5.1.44, FgaFS, festuclavine synthase) is an enzyme with systematic name festuclavine:NAD+ oxidoreductase. This enzyme catalyses the following chemical reaction
4-dimethylallyltryptophan N-methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:4-(3-methylbut-2-enyl)-L-tryptophan N-methyltransferase. This enzyme catalyses the following chemical reaction
Tropine acyltransferase is an enzyme with systematic name acyl-CoA:tropine O-acyltransferase. This enzyme catalyses the following chemical reaction
Pseudotropine acyltransferase is an enzyme with systematic name acyl-CoA:pseudotropine O-acyltransferase. This enzyme catalyses the following chemical reaction
Mycothiol synthase is an enzyme with systematic name acetyl-CoA:desacetylmycothiol O-acetyltransferase. This enzyme catalyses the following chemical reaction
UDP-2-acetamido-3-amino-2,3-dideoxy-glucuronate N-acetyltransferase is an enzyme with systematic name acetyl-CoA:UDP-2-acetamido-3-amino-2,3-dideoxy-alpha-D-glucuronate N-acetyltransferase. This enzyme catalyses the following chemical reaction
UDP-4-amino-4,6-dideoxy-N-acetyl-alpha-D-glucosamine N-acetyltransferase is an enzyme with systematic name acetyl-CoA:UDP-4-amino-4,6-dideoxy-N-acetyl-alpha-D-glucosamine N-acetyltransferase. This enzyme catalyses the following chemical reaction
Fumigaclavine A dimethylallyltransferase is an enzyme with systematic name dimethylallyl-diphosphate:fumigaclavine A dimethylallyltransferase. This enzyme catalyses the following chemical reaction

Fumigaclavine B is an ergoline compound made by certain fungi.
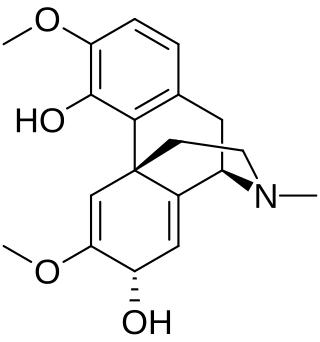
Salutaridinol is a modified benzyltetrahydroisoquinoline alkaloid with the formula C19H23NO4. It is produced in the secondary metabolism of the opium poppy Papaver somniferum (Papaveraceae) as an intermediate in the biosynthetic pathway that generates morphine. As an isoquinoline alkaloid, it is fundamentally derived from tyrosine as part of the shikimate pathway of secondary metabolism. Salutaridinol is a product of the enzyme salutaridine: NADPH 7-oxidoreductase and the substrate for the enzyme salutaridinol 7-O-acetyltransferase, which are two of the four enzymes in the morphine biosynthesis pathway that generates morphine from (R)-reticuline. Salutaridinol's unique position adjacent to two of the four enzymes in the morphine biosynthesis pathway gives it an important role in enzymatic, genetic, and synthetic biology studies of morphine biosynthesis. Salutaridinol levels are indicative of the flux through the morphine biosynthesis pathway and the efficacy of both salutaridine: NADPH 7-oxidoreductase and salutaridinol 7-O-acetyltransferase.





