Function
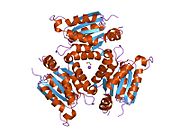
Gephyrin is a 93kDa multi-functional protein that is a component of the postsynaptic protein network of inhibitory synapses. It consists of 3 domains: N terminal G domain, C terminal E domain, and a large unstructured linker domain which connects the two. Although there are structures available for trimeric G and dimeric E domains, there is no structure available for the full length protein, which may be due to the large unstructured region which makes the protein hard to crystallize. But a recent study of the full length gephyrin by small-angle X-ray scattering shows that it predominantly forms trimers, and that because of its long linker region, it can exist in either a compact state or either of two extended states. [11]
Neuronal structure
Positive antibody staining for gephyrin at a synapse is most of the time consistent with the presence of glycine and/or GABAA receptors. Nevertheless, some exceptions can occur like in neurons of Dorsal Root Ganglions where gephyrin is absent despite the presence of GABAA receptors. [9] Gephyrin is considered a major scaffolding protein at inhibitory synapses, analogous in its function to that of PSD-95 at glutamatergic synapses. [12] [13] Gephyrin was identified by its interaction with the glycine receptor, the main receptor protein of inhibitory synapses in the spinal cord and brainstem. In addition to its interaction with the glycine receptor, recent publications have shown that gephyrin also interacts with the intracellular loop between the transmembrane helices TM3 and TM4 of alpha and beta subunits of the GABAA receptor. [14]
Gephyrin displaces GABA receptors from the GABARAP/P130 complex, then brings the receptors to the synapse. [15] Once at the synapse, the protein binds to collybistin [16] and neuroligin 2. [17] In cells, gephyrin appears to form oligomers of at least three subunits. Several splice variants have been described that prevent this oligomerization without influencing the affinity for receptors. They nevertheless affect the composition of inhibitory synapses and can even play a role in diseases like epilepsy. [18]
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.