Function
CYP24A1 is an enzyme expressed in the mitochondrion of humans and other species. It catalyzes hydroxylation reactions which lead to the degradation of 1,25-dihydroxyvitamin D3, the physiologically active form of vitamin D. Hydroxylation of the side chain produces calcitroic acid and other metabolites which are excreted in bile. [5] [6]
CYP24A1 was identified in the early 1970s and was first thought to be involved in vitamin D metabolism as the renal 25-hydroxyvitamin D3-24-hydroxylase, modifying calcifediol (25-hydroxyvitamin D) to produce 24,25-dihydroxycholecalciferol (24,25-dihydroxyvitamin D). Subsequent studies using recombinant CYP24A1 showed that it could also catalyze multiple other hydroxylation reactions at the side chain carbons known as C-24 and C-23 in both 25-OH-D3 and the active hormonal form, 1,25-(OH)2D3. It is now considered responsible for the entire five-step, 24-oxidation pathway from 1,25-(OH)2D3 producing calcitroic acid. [6]
CYP24A1 also is able to catalyze another pathway which starts with 23-hydroxylation of 1,25-(OH)2D3 and culminates in 1,25-(OH)2D3-26,23-lactone. [6]
The side chains of the ergocalciferol (vitamin D2) derivatives, 25-OH-D2 and 1,25-(OH)2D2, are also hydroxylated by CYP24A1. [6]
The structure of CYP24A1 is highly conserved between different species although the balance of functions can differ. [6] Alternatively spliced transcript variants encoding different isoforms have been found for this gene.
This enzyme plays an important role in calcium homeostasis and the vitamin D endocrine system through its regulation of the level of vitamin D3.
Interactive pathway map
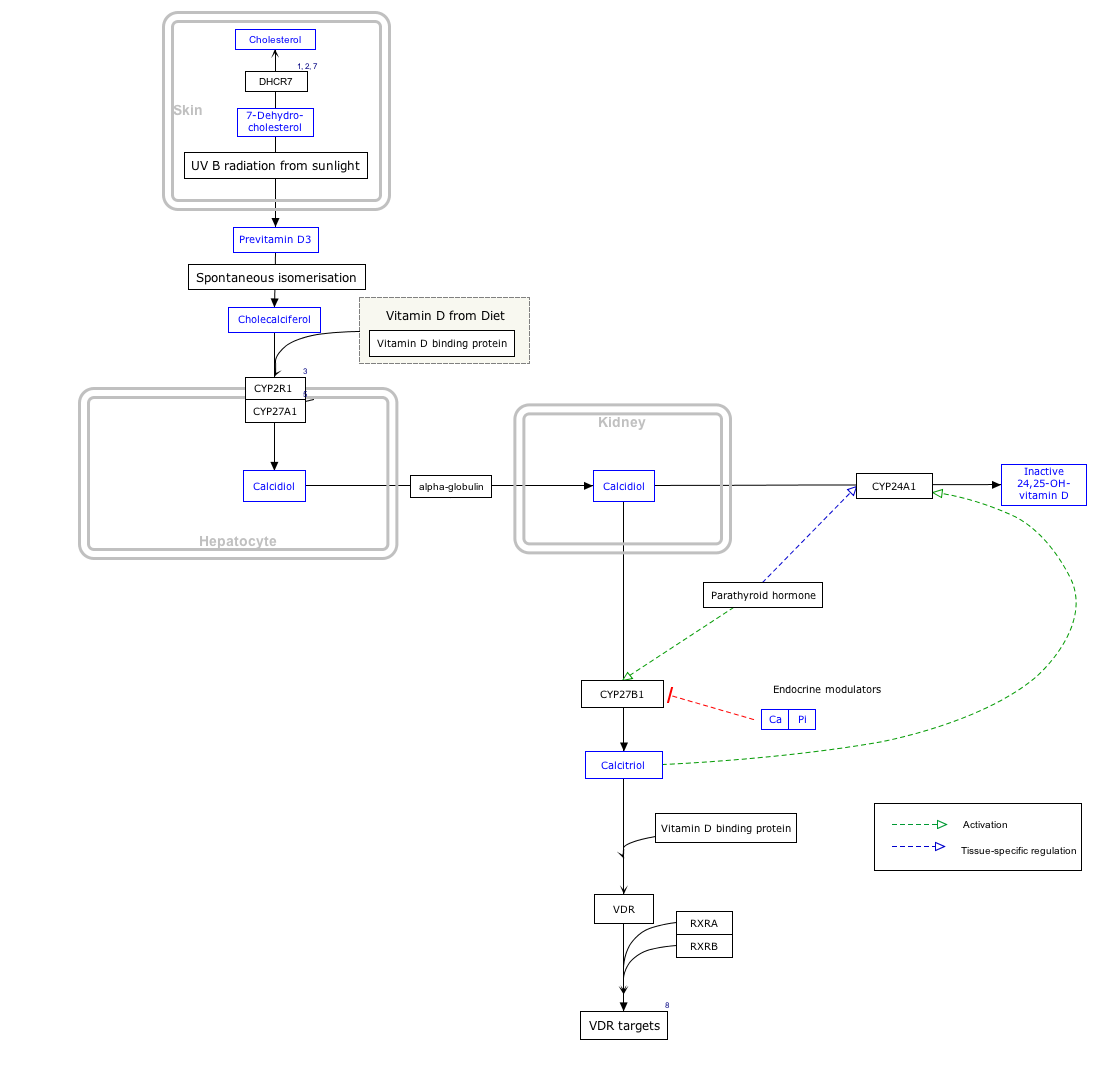
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
[[File:
|alt=Vitamin D Synthesis Pathway (
view /
edit)]]
Vitamin D Synthesis Pathway (
view /
edit)
Regulation
CYP24A1 is expressed in tissues which are considered targets for vitamin D, including kidney, intestine and bone. Transcription of the CYP24A1 gene is markedly inducible by 1,25-(OH)2D3 binding to the vitamin D receptor. [6] The gene has a strong, positive vitamin D response element in the promoter. Through regulation of CYP24A1 expression, a negative feedback control system is created to limit the effects of 1,25-(OH)2D3. [6]
PTH and FGF23 also regulate CYP24A1 gene expression. [6] Additionally, it is translationally regulated via IRES within the 5'UTR, which is responsive to an inflammatory environment. [7]
Clinical relevance
Abnormal functioning CYP24A1 is thought to be one of the causes of severe infantile hypercalcemia. [8] However, increasingly patients are also being diagnosed in adulthood, often when they present with hypercalcaemia. [9] Patients with mutations of the CYP24A1 gene have elevated serum calcium concentrations, elevated serum 1,25-(OH)2D, suppressed PTH concentrations, hypercalciuria, nephrocalcinosis, nephrolithiasis, and sometimes reduced bone density. Variations in the gene may also be found in people with renal stones. [10]
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.