Related Research Articles
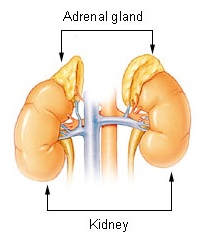
The adrenal glands are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main zones: the zona glomerulosa, the zona fasciculata and the zona reticularis.
Israel Hanukoglu is a Turkish-born Israeli scientist. He is a full professor of biochemistry and molecular biology at Ariel University and former science and technology adviser to the prime minister of Israel (1996–1999). He is founder of Israel Science and Technology Directory.

Cytochromes P450 are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown, steroid hormone synthesis, drug metabolism, and the biosynthesis of defensive compounds, fatty acids, and hormones. CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted. In almost all of the transformations that they catalyze, P450's affect hydroxylation.

Ketoconazole, sold under the brand name Nizoral among others, is an antiandrogen, antifungal, and antiglucocorticoid medication used to treat a number of fungal infections. Applied to the skin it is used for fungal skin infections such as tinea, cutaneous candidiasis, pityriasis versicolor, dandruff, and seborrheic dermatitis. Taken by mouth it is a less preferred option and only recommended for severe infections when other agents cannot be used. Other uses include treatment of excessive male-patterned hair growth in women and Cushing's syndrome.

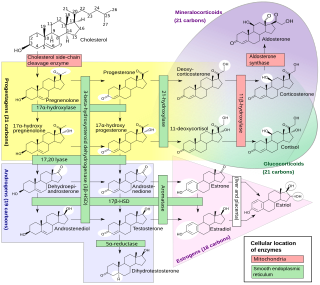
Aromatase, also called estrogen synthetase or estrogen synthase, is an enzyme responsible for a key step in the biosynthesis of estrogens. It is CYP19A1, a member of the cytochrome P450 superfamily, which are monooxygenases that catalyze many reactions involved in steroidogenesis. In particular, aromatase is responsible for the aromatization of androgens into estrogens. The enzyme aromatase can be found in many tissues including gonads, brain, adipose tissue, placenta, blood vessels, skin, and bone, as well as in tissue of endometriosis, uterine fibroids, breast cancer, and endometrial cancer. It is an important factor in sexual development.

17α-Hydroxypregnenolone is a pregnane (C21) steroid that is obtained by hydroxylation of pregnenolone at the C17α position. This step is performed by the mitochondrial cytochrome P450 enzyme 17α-hydroxylase (CYP17A1) that is present in the adrenal and gonads. Peak levels are reached in humans at the end of puberty and then decline. High levels are also achieved during pregnancy. It is also a known neuromodulator.

Cytochrome P450 17A1 is an enzyme of the hydroxylase type that in humans is encoded by the CYP17A1 gene on chromosome 10. It is ubiquitously expressed in many tissues and cell types, including the zona reticularis and zona fasciculata of the adrenal cortex as well as gonadal tissues. It has both 17α-hydroxylase and 17,20-lyase activities, and is a key enzyme in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens. More specifically, the enzyme acts upon pregnenolone and progesterone to add a hydroxyl (-OH) group at carbon 17 position (C17) of the steroid D ring, or acts upon 17α-hydroxyprogesterone and 17α-hydroxypregnenolone to split the side-chain off the steroid nucleus.

Cholesterol side-chain cleavage enzyme is commonly referred to as P450scc, where "scc" is an acronym for side-chain cleavage. P450scc is a mitochondrial enzyme that catalyzes conversion of cholesterol to pregnenolone. This is the first reaction in the process of steroidogenesis in all mammalian tissues that specialize in the production of various steroid hormones.

Steroid 21-hydroxylase is a protein that in humans is encoded by the CYP21A2 gene. The protein is an enzyme that hydroxylates steroids at the C21 position on the molecule. Naming conventions for enzymes are based on the substrate acted upon and the chemical process performed. Biochemically, this enzyme is involved in the biosynthesis of the adrenal gland hormones aldosterone and cortisol, which are important in blood pressure regulation, sodium homeostasis and blood sugar control. The enzyme converts progesterone and 17α-hydroxyprogesterone into 11-deoxycorticosterone and 11-deoxycortisol, respectively, within metabolic pathways which in humans ultimately lead to aldosterone and cortisol creation—deficiency in the enzyme may cause congenital adrenal hyperplasia.

Steroid 11β-hydroxylase, also known as steroid 11β-monooxygenase, is a steroid hydroxylase found in the zona glomerulosa and zona fasciculata of the adrenal cortex. Named officially the cytochrome P450 11B1, mitochondrial, it is a protein that in humans is encoded by the CYP11B1 gene. The enzyme is involved in the biosynthesis of adrenal corticosteroids by catalyzing the addition of hydroxyl groups during oxidation reactions.

Cytochrome P450 reductase is a membrane-bound enzyme required for electron transfer from NADPH to cytochrome P450 and other heme proteins including heme oxygenase in the endoplasmic reticulum of the eukaryotic cell.

CYP27C1 is a protein that in humans is encoded by the CYP27C1 gene. The Enzyme Commission number (EC) for this protein is EC 1.14.19.53. The full accepted name is all-trans-retinol 3,4-desaturase and the EC number 1 classifies CYP27C1 as a oxidoreductase that acts on paired donor by reducing oxygen. It is also identifiable by the UniProt code Q4G0S4.
The halloween genes are a set of genes identified in Drosophila melanogaster that influence embryonic development. All of the genes code for cytochrome P450 enzymes in the ecdysteroidogenic pathway (biosynthesis of ecdysone from cholesterol). Ecdysteroids such as 20-hydroxyecdysone and ecdysone influence many of the morphological, physiological, biochemical changes that occur during molting in insects.
An inborn error of steroid metabolism is an inborn error of metabolism due to defects in steroid metabolism.
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.
Walter L. Miller is an American endocrinologist and professor emeritus of pediatrics at the University of California, San Francisco (UCSF). Miller is expert in the field of human steroid biosynthesis and disorders of steroid metabolism. Over the past 40 years Miller's group at UCSF has described molecular basis of several metabolic disorders including, congenital adrenal hyperplasia, pseudo vitamin D dependent rickets, severe, recessive form of Ehlers-Danlos syndrome, 17,20 lyase deficiency caused by CYP17A1 defects, P450scc deficiency caused by CYP11A1 defects, P450 oxidoreductase deficiency.
The Cyp11c1 is a fish gene encoding a CYP450 enzyme, which was originally found in Zebrafish, this enzyme mainly catalyze the formation of cortisol and 11-Ketotestosterone (11-KT). 11-KT is the endogenous androgen in zebrafish. CYP11C is the orthologous to CYP11B, tetrapod's CYP11B1 evolved from CYP11C1 of fish, and CYP11B/11C are the ohonologues to CYP11A, which duplicated during 2R event.
Cytochrome P450, family 11, also known as CYP11, is a chordate cytochrome P450 monooxygenase family. This family contains many enzymes involved in steroidogenesis, such as Cholesterol side-chain cleavage enzyme (CYP11A1), Steroid 11β-hydroxylase (CYP11B1) and Aldosterone synthase (CYP11B2). CYP11 can be divided into A to E five subfamilies, and CYP11A are the ohonologues to CYP11C, which duplicated during 2R event, and the tetrapod's CYP11B evolved from CYP11C of its fish ancestors, CYP11D and F found in amphioxus. These are not the typical CYP subfamilies, which share at least 40% amino acid identity, members between CYP11A and B subfamily are only 37.5-38.8% identical, and the CYP11D and E genes seen in modern lancelet is 39% identical to catfish CYP11A1.
Cytochrome P450, family 16, also known as CYP16, is an animal cytochrome P450 monooxygenase family. This family was the last vertebrate CYP family recognized, and is absent from the mammal and zebrafish genome, but found in other fish and many invertebrates including some very old branches, such as Trichoplax and Oscarella carmela. Synteny mapping of CYP16 family members showing linkages to CYP26 family members, means the tetrapod's CYP26 may evolved from CYP16 of fish.
Cytochrome P450, family 18, also known as CYP18, is an animal cytochrome P450 family found in insect genomes. It is involved in insecticide resistance. The first member gene identified was CYP18A1, from a Drosophila melanogaster fly, acting as a dimethylnitrosamine demethylase.
References
- ↑ Uno T, Ishizuka M, Itakura T (July 2012). "Cytochrome P450 (CYP) in fish". Environmental Toxicology and Pharmacology. 34 (1): 1–13. Bibcode:2012EnvTP..34....1U. doi:10.1016/j.etap.2012.02.004. PMID 22418068.
- ↑ Parajes S, Griffin A, Taylor AE, Rose IT, Miguel-Escalada I, Hadzhiev Y, et al. (August 2013). "Redefining the initiation and maintenance of zebrafish interrenal steroidogenesis by characterizing the key enzyme cyp11a2". Endocrinology. 154 (8): 2702–11. doi: 10.1210/en.2013-1145 . PMID 23671259.
- ↑ Li N, Oakes JA, Storbeck KH, Cunliffe VT, Krone NP (February 2020). "The P450 side-chain cleavage enzyme Cyp11a2 facilitates steroidogenesis in zebrafish". The Journal of Endocrinology. 244 (2): 309–321. doi: 10.1530/JOE-19-0384 . PMID 31693487.